PASK links cellular energy metabolism with a mitotic self-renewal network to establish differentiation competence
- PMID: 37052079
- PMCID: PMC10162801
- DOI: 10.7554/eLife.81717
PASK links cellular energy metabolism with a mitotic self-renewal network to establish differentiation competence
Abstract
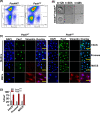
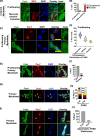
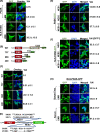
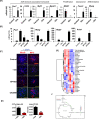
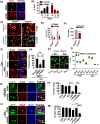
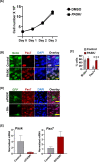
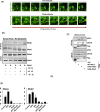
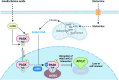
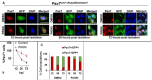
Quiescent stem cells are activated in response to a mechanical or chemical injury to their tissue niche. Activated cells rapidly generate a heterogeneous progenitor population that regenerates the damaged tissues. While the transcriptional cadence that generates heterogeneity is known, the metabolic pathways influencing the transcriptional machinery to establish a heterogeneous progenitor population remains unclear. Here, we describe a novel pathway downstream of mitochondrial glutamine metabolism that confers stem cell heterogeneity and establishes differentiation competence by countering post-mitotic self-renewal machinery. We discovered that mitochondrial glutamine metabolism induces CBP/EP300-dependent acetylation of stem cell-specific kinase, PAS domain-containing kinase (PASK), resulting in its release from cytoplasmic granules and subsequent nuclear migration. In the nucleus, PASK catalytically outcompetes mitotic WDR5-anaphase-promoting complex/cyclosome (APC/C) interaction resulting in the loss of post-mitotic Pax7 expression and exit from self-renewal. In concordance with these findings, genetic or pharmacological inhibition of PASK or glutamine metabolism upregulated Pax7 expression, reduced stem cell heterogeneity, and blocked myogenesis in vitro and muscle regeneration in mice. These results explain a mechanism whereby stem cells co-opt the proliferative functions of glutamine metabolism to generate transcriptional heterogeneity and establish differentiation competence by countering the mitotic self-renewal network via nuclear PASK.
Keywords: PASK; Pax7; Wdr5; cell biology; glutamine; human; mouse; myogenesis; self-renewal.
© 2023, Xiao, Wu, Meek et al.
Conflict of interest statement
MX, CW, GM, BK, DC, LY, SM, SD, EM, PS, AD, MG, LB, RS, CK No competing interests declared
Figures




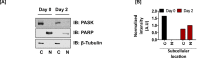

Update of
References
-
- Ahsan S, Raval MH, Ederer M, Tiwari R, Chareunsouk A, Rodgers JT. Metabolism of Glucose and Glutamine Is Critical for Skeletal Muscle Stem Cell Activation. bioRxiv. 2020 doi: 10.1101/2020.07.28.225847. - DOI
-
- Ang YS, Tsai SY, Lee DF, Monk J, Su J, Ratnakumar K, Ding J, Ge Y, Darr H, Chang B, Wang J, Rendl M, Bernstein E, Schaniel C, Lemischka IR. Wdr5 mediates self-renewal and reprogramming via the embryonic stem cell core transcriptional network. Cell. 2011;145:183–197. doi: 10.1016/j.cell.2011.03.003. - DOI - PMC - PubMed
-
- Banaszynski LA, Wen D, Dewell S, Whitcomb SJ, Lin M, Diaz N, Elsässer SJ, Chapgier A, Goldberg AD, Canaani E, Rafii S, Zheng D, Allis CD. Hira-dependent histone H3.3 deposition facilitates PRC2 recruitment at developmental loci in ES cells. Cell. 2013;155:107–120. doi: 10.1016/j.cell.2013.08.061. - DOI - PMC - PubMed
-
- Chang JS, Huypens P, Zhang Y, Black C, Kralli A, Gettys TW. Regulation of NT-PGC-1alpha subcellular localization and function by protein kinase A-dependent modulation of nuclear export by CRM1. The Journal of Biological Chemistry. 2010;285:18039–18050. doi: 10.1074/jbc.M109.083121. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

