Bi-allelic pathogenic variants in PABPC1L cause oocyte maturation arrest and female infertility
- PMID: 37052235
- PMCID: PMC10245037
- DOI: 10.15252/emmm.202217177
Bi-allelic pathogenic variants in PABPC1L cause oocyte maturation arrest and female infertility
Abstract
Oocyte maturation arrest is one of the important causes of female infertility, but the genetic factors remain largely unknown. PABPC1L, a predominant poly(A)-binding protein in Xenopus, mouse, and human oocytes and early embryos prior to zygotic genome activation, plays a key role in translational activation of maternal mRNAs. Here, we identified compound heterozygous and homozygous variants in PABPC1L that are responsible for female infertility mainly characterized by oocyte maturation arrest in five individuals. In vitro studies demonstrated that these variants resulted in truncated proteins, reduced protein abundance, altered cytoplasmic localization, and reduced mRNA translational activation by affecting the binding of PABPC1L to mRNA. In vivo, three strains of Pabpc1l knock-in (KI) female mice were infertile. RNA-sequencing analysis showed abnormal activation of the Mos-MAPK pathway in the zygotes of KI mice. Finally, we activated this pathway in mouse zygotes by injecting human MOS mRNA, and this mimicked the phenotype of KI mice. Our findings reveal the important roles of PABPC1L in human oocyte maturation and add a genetic potential candidate gene to be screened for causes of infertility.
Keywords: PABPC1L; Mos-MAPK; female infertility; mRNA translational activation; oocyte maturation arrest.
© 2023 The Authors. Published under the terms of the CC BY 4.0 license.
Conflict of interest statement
The authors declare no competing interests.
Figures
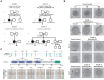
Pedigrees of the four affected families. Squares indicate male members, circles indicate female members, black circles indicate the affected individuals, question marks indicate unavailable DNA samples, and equal signs (=) indicate infertility.
The morphology of normal and affected individuals' oocytes or zygotes by light microscopy. The normal oocytes extruded PB1 (black arrowhead) and entered MII. Oocytes from individual II‐1 of family 1 were arrested at the GV or MI stage. The normal MII oocytes formed pronuclei (white arrowhead) on day 1 after ART. However, MII oocytes from individuals II‐1 and II‐2 of family 4 failed to fertilize and form pronuclei on day 1 after ART. Scale bar represents 40 μm.
Locations and conservation of variants in PABPC1L. Red arrows and green balls indicate the variants identified in this study. PABPC1L contains four different RNA recognition motifs (RRMs) and a C‐terminal protein domain (PABP).

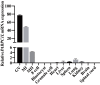
The effects of the pathogenic variants on PABPC1L expression by immunoblot in HeLa cells. Empty vector was used as the control. Vinculin was used as the loading control.
Quantitation of the Control, WT, and mutant PABPC1L protein levels shown in Fig 3A. n = 3 biological replicates. The statistics are analyzed by unpaired two‐tailed Student's t‐test. Data are shown as the mean and SD. **P < 0.005, ***P < 0.001.
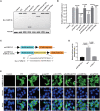
Schematic representation of dual luciferase reporter plasmid psiCHECK‐2 vectors containing the WT and mutant PABPC1L 3′UTR inserted downstream of the Renilla luciferase gene. The mutant nucleotide is marked in red.
HeLa cells were transfected with luciferase reporter constructs. Empty vector was used as the control. Relative Renilla luciferase activity was determined and normalized to Firefly luciferase activity. n = 4 biological replicates. The statistics are analyzed by unpaired two‐tailed Student's t‐test. Data are shown as the mean and SD. ****P < 0.0001.
HeLa cells were transfected with FLAG‐tagged full‐length WT or mutant PABPC1L expression plasmids and stained with an anti‐FLAG M2‐FITC antibody (green). Nuclei were labeled with Hoechst (blue). White arrowheads indicate that the p.Arg176* and p.Ile320Asnfs*122 mutant proteins were mostly mislocalized in the nucleus compared to the typical cytoplasmic localization for WT protein. Scale bar represents 20 μm.
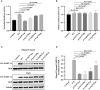
HeLa cells were transfected with FLB reporter (Firefly luciferase fused to β‐globin) together with WT or mutant PABPC1L expression plasmids, and cell extracts were assayed for Firefly luciferase activity. Empty vector was used as the control. n = 4 biological replicates. The statistics are analyzed by unpaired two‐tailed Student's t‐test. Data are shown as the mean and SD. **P < 0.01, ****P < 0.0001.
FLB mRNA levels were determined from the same cell extracts and normalized to the GAPDH mRNA level. n = 3 biological replicates. The statistics are analyzed by unpaired two‐tailed Student's t‐test. Data are shown as mean and SD. ns, not significant.
Representative immunoblots for WT and mutant PABPC1L bound to RNA in HeLa cells. HuR was used as the internal control, and Tubulin was used as the loading control.
Quantitation of the oligo‐dT–bound WT or mutant PABPC1L signal normalized to the HuR signal. n = 4 biological replicates. The statistics are analyzed by unpaired two‐tailed Student's t‐test. Data are shown as the mean and SD. **P < 0.01, ***P < 0.001, ns, not significant.
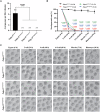
The reproductive ability of female WT and Pabpc1l KI mice. n = 6 for Pabpc1l WT/WT, Pabpc1l G97D/G97D, and Pabpc1l S137F/S137F mice. n = 3 for Pabpc1l R374Q/R374Q mice. The statistics are analyzed by unpaired two‐tailed Student's t‐test. Data are shown as mean and SD. ****P < 0.0001.
Quantification of early embryonic development at different times after IVF of superovulated oocytes from WT and Pabpc1l KI mice. The number of analyzed embryos is indicated (N). n = 4 biological replicates for Pabpc1l WT/WT, Pabpc1l G97D/G97D, and Pabpc1l S137F/S137F mice. n = 2 biological replicates for Pabpc1l R374Q/R374Q mice. The statistics are analyzed by unpaired two‐tailed Student's t‐test. Data are shown as the mean and SD. ***P < 0.001, ****P < 0.0001.
Representative images of early embryonic development at different times after IVF of superovulated oocytes from WT and Pabpc1l KI mice. Scale bar represents 100 μm.
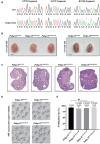
Sanger sequencing showing the homozygous mutations in Pabpc1l KI mice. Red asterisks indicate the mutation sites, and the blue asterisk indicates the synonymous mutation site.
Ovary morphology of 12‐week‐old WT and Pabpc1l KI mice. Scale bar represents 1 mm.
Histological sections of ovaries from 12‐week‐old WT and Pabpc1l KI mice were stained with hematoxylin and eosin. Scale bars represent 200 μm.
Representative images of superovulated oocytes of WT and Pabpc1l KI mice. The black arrows indicate PB1. Scale bar represents 100 μm.
Quantitative analysis of the fertilization rate of WT and Pabpc1l KI mice. The number of analyzed embryos is indicated (N). n = 8 biological replicates for Pabpc1l WT/WT, Pabpc1l G97D/G97D, and Pabpc1l S137F/S137F mice. n = 2 biological replicates for Pabpc1l R374Q/R374Q mice. The statistics are analyzed by unpaired two‐tailed Student's t‐test. Data are shown as mean and SD. ns, not significant.
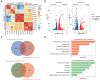
Heatmap of the Spearman correlation coefficients in the RNA‐seq data obtained from WT, G97D, and S137F zygotes.
Volcano plot of RNA‐seq data obtained from WT, G97D, and S137F zygotes. “Up” and “Down” are the upregulated and downregulated transcripts in KI zygotes compared with WT zygotes. The genes with upregulation and downregulation (abs[Fold Change] > 1.5, FDR <0.05) were labeled in red and blue, respectively.
Venn diagram showing the number of co‐upregulated and co‐downregulated genes for G97D vs. WT and S137F vs. WT.
GO analysis of co‐upregulated and co‐downregulated genes in G97D vs. WT and S137F vs. WT.
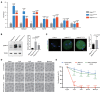
qRT–PCR results verified the expression of upregulated Mos‐MAPK pathway‐related genes and Pabpc1l in the RNA‐seq data. n = 3 biological replicates. The statistics are analyzed by unpaired two‐tailed Student's t‐test. Data are shown as the mean and SD. *P < 0.01, **P < 0.005, ***P < 0.001, ****P < 0.0001, ns, not significant.
Immunoblot analysis of pERK1/2 in WT, G97D, and S137F zygotes. Total protein from 50 zygotes was loaded in each lane. Tubulin was used as the loading control. n = 4 biological replicates. The statistics are analyzed by unpaired two‐tailed Student's t‐test. Data are shown as the mean and SD. **P < 0.005.
Immunofluorescence analysis of pERK1/2 (green) in WT and R374Q zygotes. Hoechst (blue) was co‐stained to represent pronucleus. Scale bar represents 20 μm. The number of analyzed embryos is indicated (N). n = 2 biological replicates. The statistics are analyzed by unpaired two‐tailed Student's t‐test. Data are shown as the mean and SD. ****P < 0.0001.
Representative images of early embryonic development at different times after WT zygotes were injected with H2O (Control), MOS‐WT, or MOS‐p.(Asn95Lys) cRNA. Scale bar represents 100 μm.
Quantification of early embryonic development at different times after WT zygotes were injected with H2O (Control), MOS‐WT, or MOS‐p.(Asn95Lys) cRNA. The number of analyzed embryos is indicated (N). n = 3 biological replicates. The statistics are analyzed by unpaired two‐tailed Student's t‐test. Data are shown as the mean and SD. ***P < 0.001, ns, not significant.
Comment in
-
Female infertility from oocyte maturation arrest: assembling the genetic puzzle.EMBO Mol Med. 2023 Jun 7;15(6):e17729. doi: 10.15252/emmm.202317729. Epub 2023 Apr 19. EMBO Mol Med. 2023. PMID: 37073822 Free PMC article.
References
-
- Cao Q, Zhao C, Wang C, Cai L, Xia M, Zhang X, Han J, Xu Y, Zhang J, Ling X et al (2021) The recurrent mutation in PATL2 inhibits its degradation thus causing female infertility characterized by oocyte maturation defect through regulation of the Mos‐MAPK pathway. Front Cell Dev Biol 9: 628649 - PMC - PubMed
-
- Chen B, Li B, Li D, Yan Z, Mao X, Xu Y, Mu J, Li Q, Jin L, He L et al (2017a) Novel mutations and structural deletions in TUBB8: expanding mutational and phenotypic spectrum of patients with arrest in oocyte maturation, fertilization or early embryonic development. Hum Reprod 32: 457–464 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

