Immunogenicity and safety of single booster dose of KD-414 inactivated COVID-19 vaccine in adults: An open-label, single-center, non-randomized, controlled study in Japan
- PMID: 37052247
- PMCID: PMC10114994
- DOI: 10.1080/21645515.2023.2193074
Immunogenicity and safety of single booster dose of KD-414 inactivated COVID-19 vaccine in adults: An open-label, single-center, non-randomized, controlled study in Japan
Abstract
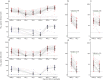
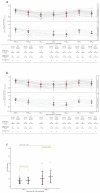
Although vaccines for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disease 2019 (COVID-19) induce effective immune responses, vaccination with booster doses is necessary because of waning immunity. We conducted an open-label, non-randomized, single-arm study in adults in Japan to assess the immunogenicity and safety of a single booster dose of the KD-414 purified whole-SARS-CoV-2-virion inactivated vaccine candidate after vaccination with a primary series of BNT162b2. The primary endpoint was serum neutralizing activity at 7 days after booster injection compared with the primary series of BNT162b2. The SARS-CoV-2-structural protein-binding antibody level and T cell response against SARS-CoV-2-Spike (S) peptides were also examined as secondary endpoints, and safety profile assessments were conducted. Twenty subjects who participated in a previous study declined an injection of KD-414 (non-KD-414 group) and received a booster dose of BNT162b2 instead. The non-KD-414 group was compared to the KD-414 group as a secondary outcome. A single dose of KD-414 induced lower serum neutralizing activity against the wild-type virus within 7 days compared to after the primary series of BNT162b2 but significantly induced anti-SARS-CoV-2-S1-receptor-binding domain-binding immunoglobulin G (IgG) antibodies and SARS-CoV-2-S peptide-specific CD4+ and CD8+ T cell responses. Local or systemic symptoms were significantly lower in the participants who received KD-414 than in those who received BNT162b2 as the third COVID-19 vaccine dose. The present data indicate that a single booster dose of KD-414 induces a substantial immune response in BNT162b2-primed individuals and has a good safety profile, thereby supporting further clinical trials to identify rational targets.
Keywords: COVID-19; COVID-19 vaccine; KD-414; SARS-CoV-2; adverse events; inactivated vaccine; neutralizing antibody; side effects; vaccine safety.
Conflict of interest statement
Kengo Sonoda is an employee of KM Biologics Co., Ltd. All rights, titles, and interests to the patent of KD-414 have been assigned to KM Biologics Co., Ltd., Kumamoto, Japan. Mugen Ujiie received research funding from KM Biologics, Co., Ltd. for this and one other study. All other authors declare that they have no competing interests related to this study.
Figures
References
-
- R&D Blue Print Team, World Health Organization . COVID-19 vaccine tracker and landscape. Website Operator; 2022. Aug 23 [accessed 2022 Aug 25]. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand....
-
- Epidemiology and prevention of vaccine-preventable diseases . The pink book: course textbook 14th edition. 2021. Washington, DC: Public Health Foundation, Centers for Disease Control and Prevention; [accessed 2022 Nov 10]. https://www.cdc.gov/vaccines/pubs/pinkbook/index.html.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous