The biology and type I/III hybrid nature of type I-D CRISPR-Cas systems
- PMID: 37052300
- PMCID: PMC10212523
- DOI: 10.1042/BCJ20220073
The biology and type I/III hybrid nature of type I-D CRISPR-Cas systems
Abstract
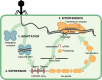
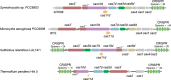
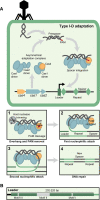
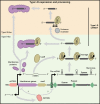
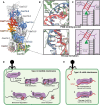
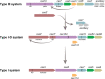
Prokaryotes have adaptive defence mechanisms that protect them from mobile genetic elements and viral infection. One defence mechanism is called CRISPR-Cas (clustered regularly interspaced short palindromic repeats and CRISPR-associated proteins). There are six different types of CRISPR-Cas systems and multiple subtypes that vary in composition and mode of action. Type I and III CRISPR-Cas systems utilise multi-protein complexes, which differ in structure, nucleic acid binding and cleaving preference. The type I-D system is a chimera of type I and III systems. Recently, there has been a burst of research on the type I-D CRISPR-Cas system. Here, we review the mechanism, evolution and biotechnological applications of the type I-D CRISPR-Cas system.
Keywords: CRISPR; RNA-binding proteins; bacteriophages; protein structure.
© 2023 The Author(s).
Conflict of interest statement
All authors are inventors on a patent application based on this research titled ‘Type I-D CRISPR–Cas Systems and Uses Thereof,’ which has been assigned Australian Patent Application No. PCT/NZ2023/050034, and the filing date of 17 March 2023.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

