Plasma Human Immunodeficiency Virus 1 RNA and CD4+ T-Cell Counts Are Determinants of Virological Nonsuppression Outcomes With Initial Integrase Inhibitor-Based Regimens: A Prospective RESPOND Cohort Study
- PMID: 37052343
- PMCID: PMC10893964
- DOI: 10.1093/cid/ciad219
Plasma Human Immunodeficiency Virus 1 RNA and CD4+ T-Cell Counts Are Determinants of Virological Nonsuppression Outcomes With Initial Integrase Inhibitor-Based Regimens: A Prospective RESPOND Cohort Study
Abstract
Background: There are conflicting data regarding baseline determinants of virological nonsuppression outcomes in persons with human immunodeficiency virus (HIV) starting antiretroviral treatment (ART). We evaluated the impact of different baseline variables in the RESPOND cohort.
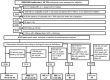
Methods: We included treatment-naive participants aged ≥18 who initiated 3-drug ART, in 2014-2020. We assessed the odds of virological suppression (VS) at weeks 48 and 96 using logistic regression. Viral blips, low-level viremia (LLV), residual viremia (RV), and virological failure (VF) rates were assessed using Cox regression.
Results: Of 4310 eligible participants, 72% started integrase strand transfer inhibitor (INSTI)-based regimens. At 48 and 96 weeks, 91.0% and 93.3% achieved VS, respectively. At 48 weeks, Kaplan-Meier estimates of rates were 9.6% for viral blips, 2.1% for LLV, 22.2% for RV, and 2.1% for VF. Baseline HIV-1 RNA levels >100 000 copies/mL and CD4+ T-cell counts ≤200/µL were negatively associated with VS at weeks 48 (adjusted odds ratio, 0.51 [95% confidence interval, .39-.68] and .40 [.27-.58], respectively) and 96 and with significantly higher rates of blips, LLV, and RV. CD4+ T-cell counts ≤200/µL were associated with higher risk of VF (adjusted hazard ratio, 3.12 [95% confidence interval, 2.02-4.83]). Results were consistent in those starting INSTIs versus other regimens and those starting dolutegravir versus other INSTIs.
Conclusions: Initial high HIV-1 RNA and low CD4+ T-cell counts are associated with lower rates of VS at 48 and 96 weeks and higher rates of viral blips, LLV, and RV. Low baseline CD4+ T-cell counts are associated with higher VF rates. These associations remain with INSTI-based and specifically with dolutegravir-based regimens. These findings suggest that the impact of these baseline determinants is independent of the ART regimen initiated.
Keywords: blip; integrase inhibitors; low-level viremia; residual viremia; virological failure.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. H. A. has received support for attending meetings from Janssen-Cilag, Gilead Sciences and ViiV Healthcare and honoraria for presentations from Gilead Sciences and ViiV Healthcare, outside the present work. A. M. has received payments or honoraria for lectures, presentations and travel support from ViiV Healthcare and Gilead Sciences and consultancy fees as an expert witness for Eiland and Bonnin, outside the submitted work. H. F. G. has received unrestricted research grants and a travel grant from Gilead Sciences; fees for data and safety monitoring board membership from Merck; consulting/advisory board membership fees from Gilead Sciences, Merck, Johnson & Johnson, Janssen, Novartis, GSK and ViiV Healthcare; and grants from the Swiss National Science Foundation, Yvonne Jacob Foundation, and the National Institutes of Health, outside the present work. C.S. reports payment or honoraria for speaking engagements from Gilead Sciences. F. W. reports membership on the advisory board for ViiV. C. L. reports grants or contracts (paid to the institution) from the German federal ministry of education and research, the Federal Joint Committee (Gemeinsamer Bundesausschuss Innovationsausschuss), the Ministerium für Kultur und Wissenschaft in Nordrhein-Westfalen, and the German Center for Infection Research; payment for speaking engagements from Pfizer, ViiV, Gilead, Novartis, MSD, and Biontech; travel support for the 2023 Conference on Retroviruses and Opportunistic Infections (CROI 2023) from Pfizer; and participation on a data safety monitoring or advisory board for ViiV, Pfizer, and Biontech. C. M. reports a research grant from Gilead (paid to the institution), consulting fees (paid to the author) from ViiV, MSD, Gilead, and Janssen, and participation on a data safety monitoring or advisory board for Corimuno. J. C. W. has received payments or honoraria for lectures from Merck Sharp & Dohme. A. S. reports grants or contracts from Gilead Sciences, participation on data safety monitoring or advisory board for GlaxoSmithKline and Gilead Sciences, and a role on the Swedish Reference Group of Antiviral Therapy. C. Smith Stephan has received grants or contracts from the Robert Koch Institute (ClinSurv Steering committee and grant) abd Gesellschaft für Internationale Zusammenarbeit (Hospital Partnership Developing Project [Esther]); consulting fees from Gilead Sciences (speaker fees and advisory board membership) and from Janssen Cilag, Merck Sharp & Dohme, ViiV Healthcare (advisory board membership); payment for expert testimony for Verwaltungsgericht Berlin; and support for attending meetings form Gilead Sciences (Conference on Retrovirus and Opportunistic Infection [American Retrovirus Conference]), Janssen-Cilag (European AIDS Conference travel grant), and AbbVie (Dagnä-Workshop travel grant), outside the present work. K. P. has received grants or contracts from ViiV Healthcare Australia and Gilead Sciences Australia, outside the present work. V. V. is an employee and shareholder of ViiV Healthcare. J. G. is an employee and shareholder of Gilead Sciences. R. M. I. has received payments for presentations in meetings and symposia as a speaker in HIV Clinical Topics (22–23 September 2022; lecture about late human immunodeficiency virus [HIV] presentation) and from the HIV Nordic Symposium (September 2022; role as chairman in a session about long-acting cabotegravir/rilpivirine [GSK]) and reports participation on an advisory board for GSK (November–December 2022) (personal consulting fee in relation to an advisory board regarding new data on dolutegravir-lamivudine and long-acting treatment with cabotegravir-rilpivirine), outside the present work. L. Y. reports other financial or nonfinancial interests with Merck Sharp & Dohme. J. M. L. has received payments or honoraria for lectures, presentations, or speakers bureau participation from Janssen-Cilag, Gilead Sciences, Thera Technologies, and ViiV Healthcare; consulting fees from and participation on a data safety monitoring or advisory board for Janssen-Cilag, Gilead Sciences, and ViiV Healthcare; and support for attending meetings from Gilead Sciences, outside the present work. A.C. reports grants or contracts (paid to institution) from ViiV Healthcare, consulting fees, payment for speaking engagements, and participation on data safety monitoring or advisory boards for Janssen Cilag, Gilead, MSD, and ViiV Healthcare (alls support paid to author). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures



References
-
- European AIDS Clinical Society . Guidelines: version 11. 1. 2022. Available at: https://www.eacsociety.org/media/guidelines-11.1_final_09-10.pdf. Accessed 14 November 2022.
-
- Laprise C, de Pokomandy A, Baril JG, et al. Virologic failure following persistent low-level viremia in a cohort of HIV-positive patients: results from 12 years of observation. Clin Infect Dis 2013; 57:1489–96. - PubMed
-
- Antiretroviral Therapy Cohort Collaboration (ART-CC); Vandenhende MA, Ingle S, May M, et al. Impact of low-level viremia on clinical and virological outcomes in treated HIV-1-infected patients. AIDS 2015; 29:373–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

