The dCache Domain of the Chemoreceptor Tlp1 in Campylobacter jejuni Binds and Triggers Chemotaxis toward Formate
- PMID: 37052512
- PMCID: PMC10294657
- DOI: 10.1128/mbio.03564-22
The dCache Domain of the Chemoreceptor Tlp1 in Campylobacter jejuni Binds and Triggers Chemotaxis toward Formate
Abstract
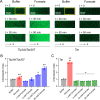
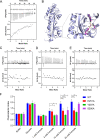
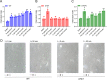
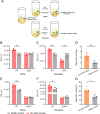
Chemotaxis is an important virulence factor in some enteric pathogens, and it is involved in the pathogenesis and colonization of the host. However, there is limited knowledge regarding the environmental signals that promote chemotactic behavior and the sensing of these signals by chemoreceptors. To date, there is no information on the ligand molecule that directly binds to and is sensed by Campylobacter jejuni Tlp1, which is a chemoreceptor with a dCache-type ligand-binding domain (LBD). dCache (double Calcium channels and chemotaxis receptor) is the largest group of sensory domains in bacteria, but the dCache-type chemoreceptor that directly binds to formate has not yet been discovered. In this study, formate was identified as a direct-binding ligand of C. jejuni Tlp1 with high sensing specificity. We used the strategy of constructing a functional hybrid receptor of C. jejuni Tlp1 and the Escherichia coli chemoreceptor Tar to screen for the potential ligand of Tlp1, with the binding of formate to Tlp1-LBD being verified using isothermal titration calorimetry. Molecular docking and experimental analyses indicated that formate binds to the membrane-proximal pocket of the dCache subdomain. Chemotaxis assays demonstrated that formate elicits robust attractant responses of the C. jejuni strain NCTC 11168, specifically via Tlp1. The chemoattraction effect of formate via Tlp1 promoted the growth of C. jejuni, especially when competing with Tlp1- or CheY-knockout strains. Our study reveals the molecular mechanisms by which C. jejuni mediates chemotaxis toward formate, and, to our knowledge, is the first report on the high-specificity binding of the dCache-type chemoreceptor to formate as well as the physiological role of chemotaxis toward formate. IMPORTANCE Chemotaxis is important for Campylobacter jejuni to colonize favorable niches in the gastrointestinal tract of its host. However, there is still a lack of knowledge about the ligand molecules for C. jejuni chemoreceptors. The dCache-type chemoreceptor, namely, Tlp1, is the most conserved chemoreceptor in C. jejuni strains; however, the direct-binding ligand(s) triggering chemotaxis has not yet been discovered. In the present study, we found that the ligand that binds directly to Tlp1-LBD with high specificity is formate. C. jejuni exhibits robust chemoattraction toward formate, primarily via Tlp1. Tlp1 is the first reported dCache-type chemoreceptor that specifically binds formate and triggers strong chemotaxis. We further demonstrated that the formate-mediated promotion of C. jejuni growth is correlated with Tlp1-mediated chemotaxis toward formate. Our work provides important insights into the mechanism and physiological function of chemotaxis toward formate and will facilitate further investigations into the involvement of microbial chemotaxis in pathogen-host interactions.
Keywords: Campylobacter jejuni; Tlp1; chemotaxis; formate; hybrid receptor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Identification of a dCache-type chemoreceptor in Campylobacter jejuni that specifically mediates chemotaxis towards methyl pyruvate.Front Microbiol. 2024 May 9;15:1400284. doi: 10.3389/fmicb.2024.1400284. eCollection 2024. Front Microbiol. 2024. PMID: 38784811 Free PMC article.
-
Diverse Sensory Repertoire of Paralogous Chemoreceptors Tlp2, Tlp3, and Tlp4 in Campylobacter jejuni.Microbiol Spectr. 2022 Dec 21;10(6):e0364622. doi: 10.1128/spectrum.03646-22. Epub 2022 Nov 14. Microbiol Spectr. 2022. PMID: 36374080 Free PMC article.
-
The dCache Chemoreceptor TlpA of Helicobacter pylori Binds Multiple Attractant and Antagonistic Ligands via Distinct Sites.mBio. 2021 Aug 31;12(4):e0181921. doi: 10.1128/mBio.01819-21. Epub 2021 Aug 3. mBio. 2021. PMID: 34340539 Free PMC article.
-
Aspartate chemosensory receptor signalling in Campylobacter jejuni.Virulence. 2010 Sep-Oct;1(5):414-7. doi: 10.4161/viru.1.5.12735. Virulence. 2010. PMID: 21178481 Review.
-
Bacterial amino acid chemotaxis: a widespread strategy with multiple physiological and ecological roles.J Bacteriol. 2024 Oct 24;206(10):e0030024. doi: 10.1128/jb.00300-24. Epub 2024 Sep 27. J Bacteriol. 2024. PMID: 39330213 Free PMC article. Review.
Cited by
-
Identification of a dCache-type chemoreceptor in Campylobacter jejuni that specifically mediates chemotaxis towards methyl pyruvate.Front Microbiol. 2024 May 9;15:1400284. doi: 10.3389/fmicb.2024.1400284. eCollection 2024. Front Microbiol. 2024. PMID: 38784811 Free PMC article.
-
Bacterial sensor evolved by decreasing complexity.Proc Natl Acad Sci U S A. 2025 Feb 4;122(5):e2409881122. doi: 10.1073/pnas.2409881122. Epub 2025 Jan 29. Proc Natl Acad Sci U S A. 2025. PMID: 39879239 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

