Pulmonary immune response regulation, genotoxicity, and metabolic reprogramming by menthol- and tobacco-flavored e-cigarette exposures in mice
- PMID: 37052522
- PMCID: PMC10230290
- DOI: 10.1093/toxsci/kfad033
Pulmonary immune response regulation, genotoxicity, and metabolic reprogramming by menthol- and tobacco-flavored e-cigarette exposures in mice
Abstract
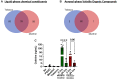
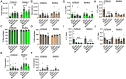
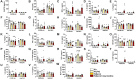
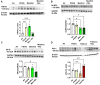
Menthol and tobacco flavors are available for almost all tobacco products, including electronic cigarettes (e-cigs). These flavors are a mixture of chemicals with overlapping constituents. There are no comparative toxicity studies of these flavors produced by different manufacturers. We hypothesized that acute exposure to menthol and tobacco-flavored e-cig aerosols induces inflammatory, genotoxicity, and metabolic responses in mouse lungs. We compared two brands, A and B, of e-cig flavors (PG/VG, menthol, and tobacco) with and without nicotine for their inflammatory response, genotoxic markers, and altered genes and proteins in the context of metabolism by exposing mouse strains, C57BL/6J (Th1-mediated) and BALB/cJ (Th2-mediated). Brand A nicotine-free menthol exposure caused increased neutrophils and differential T-lymphocyte influx in bronchoalveolar lavage fluid and induced significant immunosuppression, while brand A tobacco with nicotine elicited an allergic inflammatory response with increased Eotaxin, IL-6, and RANTES levels. Brand B elicited a similar inflammatory response in menthol flavor exposure. Upon e-cig exposure, genotoxicity markers significantly increased in lung tissue. These inflammatory and genotoxicity responses were associated with altered NLRP3 inflammasome and TRPA1 induction by menthol flavor. Nicotine decreased surfactant protein D and increased PAI-1 by menthol and tobacco flavors, respectively. Integration of inflammatory and metabolic pathway gene expression analysis showed immunometabolic regulation in T cells via PI3K/Akt/p70S6k-mTOR axis associated with suppressed immunity/allergic immune response. Overall, this study showed the comparative toxicity of flavored e-cig aerosols, unraveling potential signaling pathways of nicotine and flavor-mediated pulmonary toxicological responses, and emphasized the need for standardized toxicity testing for appropriate premarket authorization of e-cigarette products.
Keywords: ENDS; e-cigarettes; flavors; hypersensitivity; immunometabolism; menthol; nicotine; tobacco.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures






Similar articles
-
Tobacco and menthol flavored nicotine-free electronic cigarettes induced inflammation and dysregulated repair in lung fibroblast and epithelium.Respir Res. 2024 Jan 10;25(1):23. doi: 10.1186/s12931-023-02537-9. Respir Res. 2024. PMID: 38200492 Free PMC article.
-
Tobacco and Menthol flavored electronic cigarettes induced inflammation and dysregulated repair in lung fibroblast and epithelium.Res Sq [Preprint]. 2023 Jun 12:rs.3.rs-3037297. doi: 10.21203/rs.3.rs-3037297/v1. Res Sq. 2023. Update in: Respir Res. 2024 Jan 10;25(1):23. doi: 10.1186/s12931-023-02537-9. PMID: 37398084 Free PMC article. Updated. Preprint.
-
Electronic-Cigarette Vehicles and Flavoring Affect Lung Function and Immune Responses in a Murine Model.Int J Mol Sci. 2020 Aug 21;21(17):6022. doi: 10.3390/ijms21176022. Int J Mol Sci. 2020. PMID: 32825651 Free PMC article.
-
Classification, Perception, and Toxicity of Emerging Flavored Oral Nicotine Pouches.Int J Environ Res Public Health. 2023 Mar 3;20(5):4526. doi: 10.3390/ijerph20054526. Int J Environ Res Public Health. 2023. PMID: 36901533 Free PMC article. Review.
-
Electronic Cigarettes: Their Constituents and Potential Links to Asthma.Curr Allergy Asthma Rep. 2017 Oct 5;17(11):79. doi: 10.1007/s11882-017-0747-5. Curr Allergy Asthma Rep. 2017. PMID: 28983782 Free PMC article. Review.
Cited by
-
Tobacco and menthol flavored nicotine-free electronic cigarettes induced inflammation and dysregulated repair in lung fibroblast and epithelium.Respir Res. 2024 Jan 10;25(1):23. doi: 10.1186/s12931-023-02537-9. Respir Res. 2024. PMID: 38200492 Free PMC article.
-
E-cigarette exposure disrupts antitumor immunity and promotes metastasis.Front Immunol. 2024 Aug 16;15:1444020. doi: 10.3389/fimmu.2024.1444020. eCollection 2024. Front Immunol. 2024. PMID: 39221247 Free PMC article.
-
Effects of Short-term Electronic(e)-Cigarette Aerosol Exposure in the Mouse Larynx.Laryngoscope. 2024 Mar;134(3):1316-1326. doi: 10.1002/lary.31043. Epub 2023 Sep 12. Laryngoscope. 2024. PMID: 37698394 Free PMC article.
-
Clinical Impact of Vaping.Toxics. 2025 Jun 1;13(6):470. doi: 10.3390/toxics13060470. Toxics. 2025. PMID: 40559943 Free PMC article. Review.
-
Vaping and cancer metastasis: novel preclinical insights and results from the All of Us Research Program.Oncologist. 2025 Jul 4;30(7):oyaf190. doi: 10.1093/oncolo/oyaf190. Oncologist. 2025. PMID: 40589063 Free PMC article. No abstract available.
References
-
- Alam R., York J., Boyars M., Stafford S., Grant J., A., Lee J., Forsythe P., Sim T., Ida N. (1996). Increased MCP-1, RANTES, and MIP-1alpha in bronchoalveolar lavage fluid of allergic asthmatic patients. Am. J. Respir. Crit. Care Med. 153, 1398–1404. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

