A third vaccine dose equalises the levels of effectiveness and immunogenicity of heterologous or homologous COVID-19 vaccine regimens, Lyon, France, December 2021 to March 2022
- PMID: 37052679
- PMCID: PMC10103547
- DOI: 10.2807/1560-7917.ES.2023.28.15.2200746
A third vaccine dose equalises the levels of effectiveness and immunogenicity of heterologous or homologous COVID-19 vaccine regimens, Lyon, France, December 2021 to March 2022
Abstract
BackgroundTo cope with the persistence of the COVID-19 epidemic and the decrease in antibody levels following vaccination, a third dose of vaccine has been recommended in the general population. However, several vaccine regimens had been used initially for the primary vaccination course, and the heterologous Vaxzevria/Comirnaty regimen had shown better efficacy and immunogenicity than the homologous Comirnaty/Comirnaty regimen.AimWe wanted to determine if this benefit was retained after a third dose of an mRNA vaccine.MethodsWe combined an observational epidemiological study of SARS-CoV-2 infections among vaccinated healthcare workers at the University Hospital of Lyon, France, with a prospective cohort study to analyse immunological parameters before and after the third mRNA vaccine dose.ResultsFollowing the second vaccine dose, heterologous vaccination regimens were more protective against infection than homologous regimens (adjusted hazard ratio (HR) = 1.88; 95% confidence interval (CI): 1.18-3.00; p = 0.008), but this was no longer the case after the third dose (adjusted HR = 0.86; 95% CI: 0.72-1.02; p = 0.082). Receptor-binding domain-specific IgG levels and serum neutralisation capacity against different SARS-CoV-2 variants were higher after the third dose than after the second dose in the homologous regimen group, but not in the heterologous group.ConclusionThe advantage conferred by heterologous vaccination was lost after the third dose in terms of both protection and immunogenicity. Immunological measurements 1 month after vaccination suggest that heterologous vaccination induces maximal immunity after the second dose, whereas the third dose is required to reach the same level in individuals with a homologous regimen.
Keywords: SARS-CoV-2; breakthrough infection; healthcare workers; heterologous vaccine regimens; neutralisation; vaccination.
Conflict of interest statement
Figures
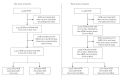


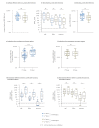
Similar articles
-
Real-world serological responses to extended-interval and heterologous COVID-19 mRNA vaccination in frail, older people (UNCoVER): an interim report from a prospective observational cohort study.Lancet Healthy Longev. 2022 Mar;3(3):e166-e175. doi: 10.1016/S2666-7568(22)00012-5. Epub 2022 Feb 23. Lancet Healthy Longev. 2022. PMID: 35224524 Free PMC article.
-
Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study.Lancet Respir Med. 2021 Nov;9(11):1255-1265. doi: 10.1016/S2213-2600(21)00357-X. Epub 2021 Aug 13. Lancet Respir Med. 2021. PMID: 34391547 Free PMC article.
-
Anti-SARS-CoV-2 spike IgG following injection of the third dose vaccine: A systematic review with meta-analysis of heterologous versus homologous vaccination.Front Public Health. 2023 Jan 12;10:960598. doi: 10.3389/fpubh.2022.960598. eCollection 2022. Front Public Health. 2023. PMID: 36711369 Free PMC article.
-
Humoral immune response after different SARS-CoV-2 vaccination regimens.BMC Med. 2022 Jan 21;20(1):31. doi: 10.1186/s12916-021-02231-x. BMC Med. 2022. PMID: 35057798 Free PMC article.
-
Binding and neutralizing antibody levels and vaccine efficacy/effectiveness compared between heterologous and homologous primary series COVID-19 vaccination: A systematic review and meta-analysis.Asian Pac J Allergy Immunol. 2022 Dec;40(4):321-336. doi: 10.12932/AP-121122-1501. Asian Pac J Allergy Immunol. 2022. PMID: 36681658
Cited by
-
Comparative effectiveness of heterologous third dose vaccine schedules against severe covid-19 during omicron predominance in Nordic countries: population based cohort analyses.BMJ. 2023 Jul 24;382:e074325. doi: 10.1136/bmj-2022-074325. BMJ. 2023. PMID: 37487623 Free PMC article.
-
The Dynamics of Antibody Titres Against SARS-CoV-2 in Vaccinated Healthcare Workers: A Systemic Literature Review.Vaccines (Basel). 2024 Dec 16;12(12):1419. doi: 10.3390/vaccines12121419. Vaccines (Basel). 2024. PMID: 39772080 Free PMC article. Review.
-
COVID-19 in City Council Civil Servants, 1 March 2020-31 January 2023: Risk of Infection, Reinfection, Vaccine Effectiveness and the Impact of Heterologous Triple Vaccination.Vaccines (Basel). 2024 Feb 28;12(3):254. doi: 10.3390/vaccines12030254. Vaccines (Basel). 2024. PMID: 38543888 Free PMC article.
-
Monitoring and active surveillance of adverse events following the booster dose of AZD1222 vaccine in people vaccinated with Sinopharm BBIBP-CorV: a cohort study.BMC Public Health. 2025 Feb 17;25(1):650. doi: 10.1186/s12889-025-21805-5. BMC Public Health. 2025. PMID: 39962455 Free PMC article.
References
-
- Liu X, Shaw RH, Stuart ASV, Greenland M, Aley PK, Andrews NJ, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398(10303):856-69. 10.1016/S0140-6736(21)01694-9 - DOI - PMC - PubMed
-
- Klemis V, Schmidt T, Schub D, Mihm J, Marx S, Abu-Omar A, et al. Comparative immunogenicity and reactogenicity of heterologous ChAdOx1-nCoV-19-priming and BNT162b2 or mRNA-1273-boosting with homologous COVID-19 vaccine regimens. Nat Commun. 2022;13(1):4710. 10.1038/s41467-022-32321-0 - DOI - PMC - PubMed
-
- Hillus D, Schwarz T, Tober-Lau P, Vanshylla K, Hastor H, Thibeault C, et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study. Lancet Respir Med. 2021;9(11):1255-65. 10.1016/S2213-2600(21)00357-X - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
