Advances in Clinical Cardiology 2022: A Summary of Key Clinical Trials
- PMID: 37052800
- PMCID: PMC10100625
- DOI: 10.1007/s12325-023-02502-4
Advances in Clinical Cardiology 2022: A Summary of Key Clinical Trials
Abstract
Introduction: Over the course of 2022, numerous key clinical trials with valuable contributions to clinical cardiology were published or presented at major international conferences. This review seeks to summarise these trials and to reflect on their clinical context.
Methods: The authors reviewed clinical trials presented at major cardiology conferences during 2022, including the American College of Cardiology (ACC), European Association for Percutaneous Cardiovascular Interventions (EuroPCR), European Society of Cardiology (ESC), Transcatheter Cardiovascular Therapeutics (TCT), American Heart Association (AHA), European Heart Rhythm Association (EHRA), Society for Cardiovascular Angiography and Interventions (SCAI), TVT-The Heart Summit (TVT) and Cardiovascular Research Technologies (CRT). Trials with a broad relevance to the cardiology community and those with potential to change current practice were included.
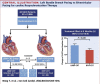
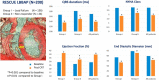
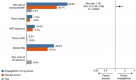
Results: A total of 93 key cardiology clinical trials were identified for inclusion. Interventional cardiology data included trials evaluating the use of new generation novel stent technology and new intravascular physiology strategies such as quantitative flow ratio (QFR) to guide revascularisation in stable and unstable coronary artery disease. New trials in acute coronary syndromes and intervention focused on long-term outcomes of optimal medical therapy (OMT), revascularisation in ischaemic dysfunction and left main (LM) intervention. Structural intervention trials included latest data on optimal timing and anticoagulation strategies in transcatheter aortic valve replacement (TAVR), in addition to expanding evidence in mitral and tricuspid valve interventions. Heart failure data included trials with sodium-glucose cotransporter 2 (SGLT2) inhibitors, iron replacement and novel drugs such as omecamtiv. Prevention trials included new data on proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors and polypill strategies. In electrophysiology, new data regarding optimal timing of ablative therapy for atrial fibrillation (AF) in addition to novel screening strategies were evaluated.
Conclusion: This article presents a summary of key clinical cardiology trials published and presented during the past year and should be of interest to both practising clinicians and researchers.
Keywords: Acute coronary syndrome; Antiplatelet therapy; Atrial fibrillation; Cardiology; Clinical trial; Electrophysiology; Heart failure; Prevention.
© 2023. Crown.
Conflict of interest statement
Ian Menown has received grants to institution, honoraria and/or conference sponsorship from Biosensors, Meril Life, and Bayer. Other authors have nothing to disclose.
Figures

Similar articles
-
Advances in Clinical Cardiology 2023: A Summary of Key Clinical Trials.Adv Ther. 2024 Jul;41(7):2606-2634. doi: 10.1007/s12325-024-02877-y. Epub 2024 May 14. Adv Ther. 2024. PMID: 38743242 Free PMC article. Review.
-
Advances in Clinical Cardiology 2024: A Summary of Key Clinical Trials.Adv Ther. 2025 Jul;42(7):3111-3140. doi: 10.1007/s12325-025-03220-9. Epub 2025 May 19. Adv Ther. 2025. PMID: 40388090 Free PMC article. Review.
-
Advances in Clinical Cardiology 2021: A Summary of Key Clinical Trials.Adv Ther. 2022 Jun;39(6):2398-2437. doi: 10.1007/s12325-022-02136-y. Epub 2022 Apr 28. Adv Ther. 2022. PMID: 35482250 Free PMC article. Review.
-
Advances in Clinical Cardiology 2020: A Summary of Key Clinical Trials.Adv Ther. 2021 May;38(5):2170-2200. doi: 10.1007/s12325-021-01711-z. Epub 2021 Apr 12. Adv Ther. 2021. PMID: 33844133 Free PMC article. Review.
-
Advances in Clinical Cardiology 2019: A Summary of Key Clinical Trials.Adv Ther. 2020 Jun;37(6):2620-2645. doi: 10.1007/s12325-020-01355-5. Epub 2020 May 2. Adv Ther. 2020. PMID: 32361851 Free PMC article. Review.
Cited by
-
Advances in Clinical Cardiology 2023: A Summary of Key Clinical Trials.Adv Ther. 2024 Jul;41(7):2606-2634. doi: 10.1007/s12325-024-02877-y. Epub 2024 May 14. Adv Ther. 2024. PMID: 38743242 Free PMC article. Review.
-
Wnt16 Promotes Vascular Smooth Muscle Contractile Phenotype and Function via Taz (Wwtr1) Activation in Male LDLR-/- Mice.Endocrinology. 2023 Dec 23;165(2):bqad192. doi: 10.1210/endocr/bqad192. Endocrinology. 2023. PMID: 38123514 Free PMC article.
-
Advances in Clinical Cardiology 2024: A Summary of Key Clinical Trials.Adv Ther. 2025 Jul;42(7):3111-3140. doi: 10.1007/s12325-025-03220-9. Epub 2025 May 19. Adv Ther. 2025. PMID: 40388090 Free PMC article. Review.
References
-
- Omerovic E, on behalf of the SCAAR investigators. Survival after PCI or CABG for left main coronary disease: a report from the Swedish Coronary Angiography and Angioplasty Registry. Presented at: TCT 2022. September 19, 2022. Boston, MA.
-
- Woodhead T, Matthews CJ, Blaxill JM, et al. Meta-analysis comparing 10-year mortality following percutaneous coronary intervention or coronary artery bypass grafting in left main stem or multivessel coronary artery disease. Am J Cardiol. 2022;174:189–191. doi: 10.1016/j.amjcard.2022.04.004. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

