Central androgen action reverses hypothalamic astrogliosis and atherogenic risk factors induced by orchiectomy and high-fat diet feeding in male mice
- PMID: 37053049
- PMCID: PMC10202485
- DOI: 10.1152/ajpendo.00059.2023
Central androgen action reverses hypothalamic astrogliosis and atherogenic risk factors induced by orchiectomy and high-fat diet feeding in male mice
Abstract
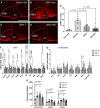
Hypogonadism in males confers elevated cardiovascular disease (CVD) risk by unknown mechanisms. Recent radiological evidence suggests that low testosterone (T) is associated with mediobasal hypothalamic (MBH) gliosis, a central nervous system (CNS) cellular response linked to metabolic dysfunction. To address mechanisms linking CNS androgen action to CVD risk, we generated a hypogonadal, hyperlipidemic mouse model with orchiectomy (ORX) combined with hepatic PCSK9 overexpression. After 4 wk of high-fat, high-sucrose diet (HFHS) consumption, despite equal body weights and glucose tolerance, androgen-deficient ORX mice had a more atherogenic lipid profile and increased liver and leukocyte inflammatory signaling compared with sham-operated control mice. Along with these early CVD risk indicators, ORX markedly amplified HFHS-induced astrogliosis in the MBH. Transcriptomic analysis further revealed that ORX and high-fat diet feeding induced upregulation of inflammatory pathways and downregulation of metabolic pathways in hypothalamic astrocytes. To interrogate the role of sex steroid signaling in the CNS in cardiometabolic risk and MBH inflammation, central infusion of T and dihydrotestosterone (DHT) was performed on ORX mice. Central DHT prevented MBH astrogliosis and reduced the liver inflammatory signaling and monocytosis induced by HFHS and ORX; T had a partial protective effect. Finally, a cross-sectional study in 41 adult men demonstrated a positive correlation between radiological evidence of MBH gliosis and plasma lipids. These findings demonstrate that T deficiency in combination with a Western-style diet promotes hypothalamic gliosis concomitant with increased atherogenic risk factors and provide supportive evidence for regulation of lipid metabolism and cardiometabolic risk determinants by the CNS action of sex steroids.NEW & NOTEWORTHY This study provides evidence that hypothalamic gliosis is a key early event through which androgen deficiency in combination with a Western-style diet might lead to cardiometabolic dysregulation in males. Furthermore, this work provides the first evidence in humans of a positive association between hypothalamic gliosis and LDL-cholesterol, advancing our knowledge of CNS influences on CVD risk progression.
Keywords: astrocyte; cardiovascular risk; hypogonadism; hypothalamic gliosis; testosterone.
Conflict of interest statement
T. Monfeuga, A. Chandran, and T. H. Meek are employees of Novo Nordisk Ltd. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

