Eye Drop Adherence With an Eye Drop Bottle Cap Monitor
- PMID: 37053080
- PMCID: PMC10132997
- DOI: 10.1097/IJG.0000000000002166
Eye Drop Adherence With an Eye Drop Bottle Cap Monitor
Abstract
Prcis: An eye drop bottle cap monitor with audio and visual alarms measured eye drop adherence in 50 subjects with glaucoma. Baseline adherence rates were too high to test if the alarms could improve adherence.
Purpose: To determine if an eye drop bottle cap monitor can measure and improve adherence.
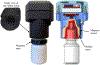
Materials and methods: The Devers Drop Device (D3, Universal Adherence LLC) was designed to measure eye drop adherence by detecting bottle cap removal and replacement, and it can provide text, visual and audio alerts when a medication is due. In Stage 1, we determined baseline adherence for 50 subjects using a nightly eye drop over a 25-day period. Subjects with less than 90% baseline adherence were eligible for Stage 2. In Stage 2, we randomized subjects to receive either no reminder or automated D3 alerts for their nightly eye drop over a subsequent 25-day period. We defined adherence as the proportion of drops administered within 3 hours of the subjects' scheduled dosing time. Subjects completed 3 questions regarding satisfaction with the device and willingness to pay.
Results: The D3 monitor remained attached to the eye drop bottle cap for the duration of the study and collected adherence data in all 50 patients. In Stage 1, the mean adherence rate was 90 ± 18% (range 32-100%). Forty (80%) subjects had an adherence rate greater than 90%. Adherence rates were too high in Stage 1 to adequately test the effects of reminders in Stage 2. Ninety-eight percent (49/50) and 96% (48/50) of subjects agreed "the device always stayed attached to the bottle cap" and "I was able to use the device to take the drops", respectively. Patients would pay $61±83 (range $0-400) for a similar device to improve adherence.
Conclusions: The D3 can measure eye drop adherence. Research subjects reported high satisfaction and willingness to pay for an eye drop bottle cap monitor. Glaucoma patients have high adherence when they are being monitored, and future studies with research subjects screened for poor adherence may further determine the benefit of electronic monitoring of adherence with and without electronic reminders.
Copyright © 2023 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
Disclosure: R.M.K. and S.L.M.: Commercial Relationship(s); Universal Adherence LLC: Code I (Personal Financial Interest); Universal Adherence LLC—(U.S. Patent No. US 11,045,349): Code P (Patent); National Institutes of Health: Code F (Financial Support). Due to their conflict of interest, R.M.K. and S.L.M. had no direct access to study data. The remaining authors have no conflicts of interest.
Figures
References
-
- GBD 2019 Blindness and Vision Impairment Collaborators. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. The Lancet. Global health. 2021;9(2):e144–e160. - PMC - PubMed
-
- Friedman DS, Nordstrom B, Mozaffari E, Quigley HA. Glaucoma management among individuals enrolled in a single comprehensive insurance plan. Ophthalmology. Sep 2005;112(9):1500–1504. - PubMed
-
- Okeke CO, Quigley HA, Jampel HD, et al. Adherence with topical glaucoma medication monitored electronically the Travatan Dosing Aid study. Ophthalmology. Feb 2009;116(2):191–199. - PubMed
-
- Barker GT, Cook PF, Schmiege SJ, Kahook MY, Kammer JA, Mansberger SL. Psychometric properties of the Glaucoma Treatment Compliance Assessment Tool in a multicenter trial. Am J Ophthalmol. Jun 2015;159(6):1092–1099 e1092. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

