Validation of an orthotopic non-small cell lung cancer mouse model, with left or right tumor growths, to use in conformal radiotherapy studies
- PMID: 37053154
- PMCID: PMC10101527
- DOI: 10.1371/journal.pone.0284282
Validation of an orthotopic non-small cell lung cancer mouse model, with left or right tumor growths, to use in conformal radiotherapy studies
Abstract
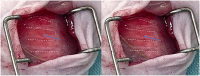
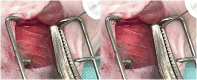
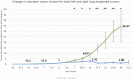
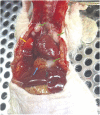
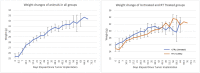
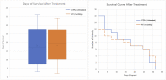
Orthotopic non-small cell lung cancer (NSCLC) mice models are important for establishing translatability of in vitro results. However, most orthotopic lung models do not produce localized tumors treatable by conformal radiotherapy (RT). Here we report on the performance of an orthotopic mice model featuring conformal RT treatable tumors following either left or right lung tumor cell implantation. Athymic Nude mice were surgically implanted with H1299 NSCLC cell line in either the left or right lung. Tumor development was tracked bi-weekly using computed tomography (CT) imaging. When lesions reached an appropriate size for treatment, animals were separated into non-treatment (control group) and RT treated groups. Both RT treated left and right lung tumors which were given a single dose of 20 Gy of 225 kV X-rays. Left lung tumors were treated with a two-field parallel opposed plan while right lung tumors were treated with a more conformal four-field plan to assess tumor control. Mice were monitored for 30 days after RT or after tumor reached treatment size for non-treatment animals. Treatment images from the left and right lung tumor were also used to assess the dose distribution for four distinct treatment plans: 1) Two sets of perpendicularly staggered parallel opposed fields, 2) two fields positioned in the anterior-posterior and posterior-anterior configuration, 3) an 180° arc field from 0° to 180° and 4) two parallel opposed fields which cross through the contralateral lung. Tumor volumes and changes throughout the follow-up period were tracked by three different types of quantitative tumor size approximation and tumor volumes derived from contours. Ultimately, our model generated delineable and conformal RT treatable tumor following both left and right lung implantation. Similarly consistent tumor development was noted between left and right models. We were also able to demonstrate that a single 20 Gy dose of 225 kV X-rays applied to either the right or left lung tumor models had similar levels of tumor control resulting in similar adverse outcomes and survival. And finally, three-dimensional tumor approximation featuring volume computed from the measured length across three perpendicular axes gave the best approximation of tumor volume, most closely resembled tumor volumes obtained with contours.
Copyright: © 2023 Wang et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

