The active zone protein Clarinet regulates synaptic sorting of ATG-9 and presynaptic autophagy
- PMID: 37053235
- PMCID: PMC10101500
- DOI: 10.1371/journal.pbio.3002030
The active zone protein Clarinet regulates synaptic sorting of ATG-9 and presynaptic autophagy
Abstract
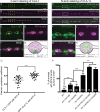
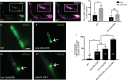
Autophagy is essential for cellular homeostasis and function. In neurons, autophagosome biogenesis is temporally and spatially regulated to occur near presynaptic sites, in part via the trafficking of autophagy transmembrane protein ATG-9. The molecules that regulate autophagy by sorting ATG-9 at synapses remain largely unknown. Here, we conduct forward genetic screens at single synapses of C. elegans neurons and identify a role for the long isoform of the active zone protein Clarinet (CLA-1L) in regulating sorting of autophagy protein ATG-9 at synapses, and presynaptic autophagy. We determine that disrupting CLA-1L results in abnormal accumulation of ATG-9 containing vesicles enriched with clathrin. The ATG-9 phenotype in cla-1(L) mutants is not observed for other synaptic vesicle proteins, suggesting distinct mechanisms that regulate sorting of ATG-9-containing vesicles and synaptic vesicles. Through genetic analyses, we uncover the adaptor protein complexes that genetically interact with CLA-1 in ATG-9 sorting. We also determine that CLA-1L extends from the active zone to the periactive zone and genetically interacts with periactive zone proteins in ATG-9 sorting. Our findings reveal novel roles for active zone proteins in the sorting of ATG-9 and in presynaptic autophagy.
Copyright: © 2023 Xuan et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
The active zone protein CLA-1 (Clarinet) bridges two subsynaptic domains to regulate presynaptic sorting of ATG-9.Autophagy. 2023 Oct;19(10):2807-2808. doi: 10.1080/15548627.2023.2229227. Epub 2023 Jun 30. Autophagy. 2023. PMID: 37389488 Free PMC article.
-
Presynaptic autophagy is coupled to the synaptic vesicle cycle via ATG-9.Neuron. 2022 Mar 2;110(5):824-840.e10. doi: 10.1016/j.neuron.2021.12.031. Epub 2022 Jan 21. Neuron. 2022. PMID: 35065714 Free PMC article.
-
Clarinet (CLA-1), a novel active zone protein required for synaptic vesicle clustering and release.Elife. 2017 Nov 21;6:e29276. doi: 10.7554/eLife.29276. Elife. 2017. PMID: 29160205 Free PMC article.
-
Neurons Specialize in Presynaptic Autophagy: A Perspective to Ameliorate Neurodegeneration.Mol Neurobiol. 2025 Feb;62(2):2626-2640. doi: 10.1007/s12035-024-04399-8. Epub 2024 Aug 14. Mol Neurobiol. 2025. PMID: 39141193 Review.
-
Molecular organization and assembly of the presynaptic active zone of neurotransmitter release.Results Probl Cell Differ. 2006;43:49-68. doi: 10.1007/400_012. Results Probl Cell Differ. 2006. PMID: 17068967 Review.
Cited by
-
Monitoring of activity-driven trafficking of endogenous synaptic proteins through proximity labeling.PLoS Biol. 2024 Oct 28;22(10):e3002860. doi: 10.1371/journal.pbio.3002860. eCollection 2024 Oct. PLoS Biol. 2024. PMID: 39466808 Free PMC article.
-
Emerging roles of ATG9/ATG9A in autophagy: implications for cell and neurobiology.Autophagy. 2024 Nov;20(11):2373-2387. doi: 10.1080/15548627.2024.2384349. Epub 2024 Aug 4. Autophagy. 2024. PMID: 39099167 Free PMC article. Review.
-
Presynaptic Autophagy and the Connection With Neurotransmission.Front Cell Dev Biol. 2021 Dec 17;9:790721. doi: 10.3389/fcell.2021.790721. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34988081 Free PMC article. Review.
-
Turning garbage into gold: Autophagy in synaptic function.Curr Opin Neurobiol. 2025 Feb;90:102937. doi: 10.1016/j.conb.2024.102937. Epub 2024 Dec 12. Curr Opin Neurobiol. 2025. PMID: 39667255 Review.
-
Organization of Presynaptic Autophagy-Related Processes.Front Synaptic Neurosci. 2022 Mar 17;14:829354. doi: 10.3389/fnsyn.2022.829354. eCollection 2022. Front Synaptic Neurosci. 2022. PMID: 35368245 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

