Sequential bilateral accelerated theta burst stimulation in adolescents with suicidal ideation associated with major depressive disorder: Protocol for a randomized controlled trial
- PMID: 37053246
- PMCID: PMC10101506
- DOI: 10.1371/journal.pone.0280010
Sequential bilateral accelerated theta burst stimulation in adolescents with suicidal ideation associated with major depressive disorder: Protocol for a randomized controlled trial
Abstract
Background: Suicide is a leading cause of death in adolescents worldwide. Previous research findings suggest that suicidal adolescents with depression have pathophysiological dorsolateral prefrontal cortex (DLPFC) deficits in γ-aminobutyric acid neurotransmission. Interventions with transcranial magnetic stimulation (TMS) directly address these underlying pathophysiological deficits in the prefrontal cortex. Theta burst stimulation (TBS) is newer dosing approach for TMS. Accelerated TBS (aTBS) involves administering multiple sessions of TMS daily as this dosing may be more efficient, tolerable, and rapid acting than standard TMS.
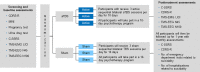
Materials and methods: This is a randomized, double-blind, sham-controlled trial of sequential bilateral aTBS in adolescents with major depressive disorder (MDD) and suicidal ideation. Three sessions are administered daily for 10 days. During each session, continuous TBS is administered first to the right DPFC, in which 1,800 pulses are delivered continuously over 120 seconds. Then intermittent TBS is applied to the left DPFC, in which 1,800 pulses are delivered in 2-second bursts and repeated every 10 seconds for 570 seconds. The TBS parameters were adopted from prior research, with 3-pulse, 50-Hz bursts given every 200 ms (at 5 Hz) with an intensity of 80% active motor threshold. The comparison group will receive 3 daily sessions of bilateral sham TBS treatment for 10 days. All participants will receive the standard of care for patients with depression and suicidal ideation including daily psychotherapeutic skill sessions. Long-interval intracortical inhibition (LICI) biomarkers will be measured before and after treatment. Exploratory measures will be collected with TMS and electroencephalography for biomarker development.
Discussion: This is the first known randomized controlled trial to examine the efficacy of sequential bilateral aTBS for treating suicidal ideation in adolescents with MDD. Results from this study will also provide opportunities to further understand the neurophysiological and molecular mechanisms of suicidal ideation in adolescents.
Trial registration: Investigational device exemption (IDE) Number: G200220, ClinicalTrials.gov (ID: NCT04701840). Registered August 6, 2020. https://clinicaltrials.gov/ct2/show/NCT04502758?term=NCT04701840&draw=2&rank=1.
Copyright: © 2023 Yuruk et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
Dr Croarkin has received research grant support from Neuronetics Inc, NeoSync Inc, and Pfizer Inc. He has received in-kind support (equipment, supplies, and genotyping) for research studies from Assurex Health Inc, Neuronetics Inc, and MagVenture Inc. He has consulted for Engrail Therapeutics Inc, Myriad Neuroscience, Procter & Gamble, and Sunovion. Dr Vande Voort has received in-kind support (supplies and genotyping) from Assurex Health Inc. The other authors declare that they have no competing interests.
Figures

Similar articles
-
A naturalistic study comparing the efficacy of unilateral and bilateral sequential theta burst stimulation in treating major depression - the U-B-D study protocol.BMC Psychiatry. 2023 Oct 10;23(1):739. doi: 10.1186/s12888-023-05243-4. BMC Psychiatry. 2023. PMID: 37817124 Free PMC article.
-
Effect of intermittent theta burst stimulation on suicidal ideation and depressive symptoms in adolescent depression with suicide attempt: A randomized sham-controlled study.J Affect Disord. 2023 Mar 15;325:618-626. doi: 10.1016/j.jad.2023.01.061. Epub 2023 Jan 19. J Affect Disord. 2023. PMID: 36682694 Clinical Trial.
-
Adjunctive duration-doubled transcranial direct current stimulation for the treatment of depressive patients with suicidal ideation: study protocol for a double-blind, randomized, sham-controlled trial.Trials. 2024 Jan 2;25(1):15. doi: 10.1186/s13063-023-07858-0. Trials. 2024. PMID: 38167178 Free PMC article.
-
Bilateral theta burst stimulation for patients with acute unipolar or bipolar depressive episodes: A systematic review of randomized controlled studies.J Affect Disord. 2023 Nov 1;340:575-582. doi: 10.1016/j.jad.2023.08.065. Epub 2023 Aug 12. J Affect Disord. 2023. PMID: 37579881
-
Theta-burst stimulation: a new form of TMS treatment for depression?Depress Anxiety. 2015 Mar;32(3):182-92. doi: 10.1002/da.22335. Epub 2014 Nov 28. Depress Anxiety. 2015. PMID: 25450537 Review.
Cited by
-
Psychoneuroimmunoendocrinological and neuroanatomical basis of suicidal behavior: potential therapeutic strategies with a focus on transcranial magnetic stimulation (TMS).Brain Behav Immun Health. 2025 Apr 21;46:101002. doi: 10.1016/j.bbih.2025.101002. eCollection 2025 Jul. Brain Behav Immun Health. 2025. PMID: 40337353 Free PMC article.
-
Repetitive transcranial magnetic stimulation frequency effects on suicidal ideation in adolescents with major depressive disorder.J Affect Disord. 2025 Aug 15;383:101-107. doi: 10.1016/j.jad.2025.04.112. Epub 2025 Apr 23. J Affect Disord. 2025. PMID: 40280439 Clinical Trial.
References
-
- World Health Organization. Preventing suicide: a global imperative. Geneva: World Health Organization; 2014. Available from: https://www.who.int/publications/i/item/9789241564779.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

