Mice lacking nucleotide sugar transporter SLC35A3 exhibit lethal chondrodysplasia with vertebral anomalies and impaired glycosaminoglycan biosynthesis
- PMID: 37053259
- PMCID: PMC10101523
- DOI: 10.1371/journal.pone.0284292
Mice lacking nucleotide sugar transporter SLC35A3 exhibit lethal chondrodysplasia with vertebral anomalies and impaired glycosaminoglycan biosynthesis
Abstract
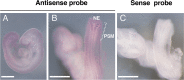
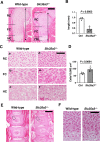
SLC35A3 is considered an uridine diphosphate N-acetylglucosamine (UDP-GlcNAc) transporter in mammals and regulates the branching of N-glycans. A missense mutation in SLC35A3 causes complex vertebral malformation (CVM) in cattle. However, the biological functions of SLC35A3 have not been fully clarified. To address these issues, we have established Slc35a3-/-mice using CRISPR/Cas9 genome editing system. The generated mutant mice were perinatal lethal and exhibited chondrodysplasia recapitulating CVM-like vertebral anomalies. During embryogenesis, Slc35a3 mRNA was expressed in the presomitic mesoderm of wild-type mice, suggesting that SLC35A3 transports UDP-GlcNAc used for the sugar modification that is essential for somite formation. In the growth plate cartilage of Slc35a3-/-embryos, extracellular space was drastically reduced, and many flat proliferative chondrocytes were reshaped. Proliferation, apoptosis and differentiation were not affected in the chondrocytes of Slc35a3-/-mice, suggesting that the chondrodysplasia phenotypes were mainly caused by the abnormal extracellular matrix quality. Because these histological abnormalities were similar to those observed in several mutant mice accompanying the impaired glycosaminoglycan (GAG) biosynthesis, GAG levels were measured in the spine and limbs of Slc35a3-/-mice using disaccharide composition analysis. Compared with control mice, the amounts of heparan sulfate, keratan sulfate, and chondroitin sulfate/dermatan sulfate, were significantly decreased in Slc35a3-/-mice. These findings suggest that SLC35A3 regulates GAG biosynthesis and the chondrodysplasia phenotypes were partially caused by the decreased GAG synthesis. Hence, Slc35a3-/- mice would be a useful model for investigating the in vivo roles of SLC35A3 and the pathological mechanisms of SLC35A3-associated diseases.
Copyright: © 2023 Saito et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Biosynthesis of GlcNAc-rich N- and O-glycans in the Golgi apparatus does not require the nucleotide sugar transporter SLC35A3.J Biol Chem. 2020 Nov 27;295(48):16445-16463. doi: 10.1074/jbc.RA119.012362. Epub 2020 Sep 16. J Biol Chem. 2020. PMID: 32938718 Free PMC article.
-
A missense mutation in the bovine SLC35A3 gene, encoding a UDP-N-acetylglucosamine transporter, causes complex vertebral malformation.Genome Res. 2006 Jan;16(1):97-105. doi: 10.1101/gr.3690506. Epub 2005 Dec 12. Genome Res. 2006. PMID: 16344554 Free PMC article.
-
UDP-N-acetylglucosamine transporter (SLC35A3) regulates biosynthesis of highly branched N-glycans and keratan sulfate.J Biol Chem. 2013 Jul 26;288(30):21850-60. doi: 10.1074/jbc.M113.460543. Epub 2013 Jun 13. J Biol Chem. 2013. PMID: 23766508 Free PMC article.
-
Congenital Disorders of Deficiency in Glycosaminoglycan Biosynthesis.Front Genet. 2021 Sep 3;12:717535. doi: 10.3389/fgene.2021.717535. eCollection 2021. Front Genet. 2021. PMID: 34539746 Free PMC article. Review.
-
Chondrodysplasia of gene knockout mice for aggrecan and link protein.Glycoconj J. 2002 May-Jun;19(4-5):269-73. doi: 10.1023/A:1025344332099. Glycoconj J. 2002. PMID: 12975605 Review.
Cited by
-
Genetic variability in proteoglycan biosynthetic genes reveals new facets of heparan sulfate diversity.Essays Biochem. 2024 Dec 4;68(4):555-578. doi: 10.1042/EBC20240106. Essays Biochem. 2024. PMID: 39630030 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

