Cardiovascular Calcification Heterogeneity in Chronic Kidney Disease
- PMID: 37053279
- PMCID: PMC10097496
- DOI: 10.1161/CIRCRESAHA.123.321760
Cardiovascular Calcification Heterogeneity in Chronic Kidney Disease
Abstract
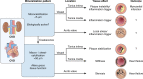
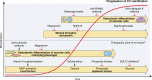
Patients with chronic kidney disease (CKD) exhibit tremendously elevated risk for cardiovascular disease, particularly ischemic heart disease, due to premature vascular and cardiac aging and accelerated ectopic calcification. The presence of cardiovascular calcification associates with increased risk in patients with CKD. Disturbed mineral homeostasis and diverse comorbidities in these patients drive increased systemic cardiovascular calcification in different manifestations with diverse clinical consequences, like plaque instability, vessel stiffening, and aortic stenosis. This review outlines the heterogeneity in calcification patterning, including mineral type and location and potential implications on clinical outcomes. The advent of therapeutics currently in clinical trials may reduce CKD-associated morbidity. Development of therapeutics for cardiovascular calcification begins with the premise that less mineral is better. While restoring diseased tissues to a noncalcified homeostasis remains the ultimate goal, in some cases, calcific mineral may play a protective role, such as in atherosclerotic plaques. Therefore, developing treatments for ectopic calcification may require a nuanced approach that considers individual patient risk factors. Here, we discuss the most common cardiac and vascular calcification pathologies observed in CKD, how mineral in these tissues affects function, and the potential outcomes and considerations for therapeutic strategies that seek to disrupt the nucleation and growth of mineral. Finally, we discuss future patient-specific considerations for treating cardiac and vascular calcification in patients with CKD-a population in need of anticalcification therapies.
Keywords: aortic valve stenosis; minerals; renal insufficiency, chronic; therapeutics; vascular calcification.
Conflict of interest statement
None.
Figures
Similar articles
-
Pathophysiology and treatment of cardiovascular disease in pediatric chronic kidney disease.Pediatr Nephrol. 2019 Jan;34(1):1-10. doi: 10.1007/s00467-017-3798-x. Epub 2017 Sep 22. Pediatr Nephrol. 2019. PMID: 28939921 Review.
-
Mineral metabolism and cardiovascular disease in CKD.Clin Exp Nephrol. 2017 Mar;21(Suppl 1):53-63. doi: 10.1007/s10157-016-1363-8. Epub 2017 Jan 6. Clin Exp Nephrol. 2017. PMID: 28062938 Review.
-
Current and potential therapeutic strategies for the management of vascular calcification in patients with chronic kidney disease including those on dialysis.Semin Dial. 2018 Sep;31(5):487-499. doi: 10.1111/sdi.12710. Epub 2018 May 7. Semin Dial. 2018. PMID: 29733462 Review.
-
[Vascular Calcification - Pathological Mechanism and Clinical Application - . Vascular calcification in chronic kidney disease-mineral and bone disorder (CKD-MBD)].Clin Calcium. 2015 May;25(5):645-53. Clin Calcium. 2015. PMID: 25926567 Review. Japanese.
-
Mechanistic insights into CKD-MBD-related vascular calcification and its clinical implications.Life Sci. 2022 Dec 15;311(Pt B):121148. doi: 10.1016/j.lfs.2022.121148. Epub 2022 Nov 3. Life Sci. 2022. PMID: 36336124 Review.
Cited by
-
Characteristics of calcified nodule attributable to culprit lesion in acute coronary syndrome: A systematic review and meta-analysis.iScience. 2024 Jun 22;27(7):110351. doi: 10.1016/j.isci.2024.110351. eCollection 2024 Jul 19. iScience. 2024. PMID: 39092174 Free PMC article.
-
FOXO1 regulates RUNX2 ubiquitination through SMURF2 in calcific aortic valve disease.Redox Biol. 2024 Jul;73:103215. doi: 10.1016/j.redox.2024.103215. Epub 2024 May 27. Redox Biol. 2024. PMID: 38810422 Free PMC article.
-
Evaluation of Individual Cardiovascular Risk in Pre-Dialysis CKD Patients by Using the Ratio of Calcium-Phosphorus Product to Estimated Glomerular Filtration Rate (Ca × P/eGFR).Biomedicines. 2025 Jan 19;13(1):235. doi: 10.3390/biomedicines13010235. Biomedicines. 2025. PMID: 39857818 Free PMC article.
-
Endothelial injury is one of the risk factors for the progression of vascular calcification in patients receiving maintenance dialysis.Ren Fail. 2025 Dec;47(1):2456690. doi: 10.1080/0886022X.2025.2456690. Epub 2025 Jan 26. Ren Fail. 2025. PMID: 39865575 Free PMC article.
-
[Arterial mediacalcinosis in patients with diabetes mellitus: etiopathogenetic and histopathological aspects].Probl Endokrinol (Mosk). 2024 Apr 24;71(1):50-59. doi: 10.14341/probl13360. Probl Endokrinol (Mosk). 2024. PMID: 40089885 Free PMC article. Review. Russian.
References
-
- Matsushita K, Ballew SH, Wang AY, Kalyesubula R, Schaeffner E, Agarwal R. Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. Nat Rev Nephrol. 2022;18:696–707. doi: 10.1038/s41581-022-00616-6 - PubMed
-
- Akbari A, Swedko PJ, Clark HD, Hogg W, Lemelin J, Magner P, Moore L, Ooi D. Detection of chronic kidney disease with laboratory reporting of estimated glomerular filtration rate and an educational program. Arch Intern Med. 2004;164:1788–1792. doi: 10.1001/archinte.164.16.1788 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

