Grape seed meal by-product is able to counteract oxidative stress induced by lipopolysaccharide and dextran sulphate in IPEC cells and piglets after weaning
- PMID: 37053301
- PMCID: PMC10101422
- DOI: 10.1371/journal.pone.0283607
Grape seed meal by-product is able to counteract oxidative stress induced by lipopolysaccharide and dextran sulphate in IPEC cells and piglets after weaning
Abstract
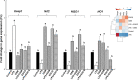
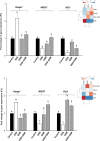
Oxidative stress is a pivotal factor in the pathogenesis of intestinal inflammation, leading to cellular damage and tissue injury. Natural antioxidants compounds found in agro-industrial by-products have proven their effectiveness in treatment of intestinal inflammation and oxidative stress, exhibiting many favourable effects. The aim of this study was to evaluate the capacity of a grape seed meal byproduct (GSM) to counteract the effects induced by E. coli lipopolysaccharide (LPS, 5μg/ml) in vitro on IPEC-1 cells and by dextran sulphate sodium (DSS, 1g/b.w./day) in vivo on piglets after weaning. Reactive oxygen species (ROS), pro-oxidant markers (malondialdehyde MDA, thiobarbituric acid reactive substances TBARS, protein carbonyl, DNA oxidative damage) antioxidant enzymes (catalase -CAT, superoxide dismutase -SOD, glutathione peroxidase -GPx, endothelial and inducible nitric oxide synthases -eNOS and iNOS) and several important components of Keap1/Nrf2 signalling pathway were analysed in IPEC-1 cells as well as in piglet's colon and lymph nodes. Our results demonstrated that GSM extract or 8% dietary GSM showed anti-oxidant properties counteracting the pro-oxidant response (ROS, MDA-TBARS, protein carbonyl, DNA/RNA damage) induced by LPS or DSS and restoring the levels of endogenous antioxidant enzymes, including CAT, SOD, GPx, eNOS and iNOS in colon and mesenteric lymph nodes. These beneficial effects were modulated via Nrf2 signalling pathway in both in vitro and in vivo studies.
Copyright: © 2023 Pistol et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
-
- Alemany-Cosme E., Saez-Gonzalez E, Moret I., Mateos B., Iborra M., Nos P., et al. Oxidative Stress in the Pathogenesis of Crohn’s Disease and the Interconnection with Immunological Response, Microbiota, External Environmental Factors, and Epigenetics. Antioxidants (Basel). 2021;10(64):21. doi: 10.3390/antiox10010064 - DOI - PMC - PubMed
-
- Wang Y, Chen Y, Zhang X, Lu Y, Chen H. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: A review. Journal of Functional Foods. 2020;75:17. doi: 10.1016/j.jff.2020.104248 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

