Trametinib in Patients With NF1-, GNAQ-, or GNA11-Mutant Tumors: Results From the NCI-MATCH ECOG-ACRIN Trial (EAY131) Subprotocols S1 and S2
- PMID: 37053535
- PMCID: PMC10309549
- DOI: 10.1200/PO.22.00421
Trametinib in Patients With NF1-, GNAQ-, or GNA11-Mutant Tumors: Results From the NCI-MATCH ECOG-ACRIN Trial (EAY131) Subprotocols S1 and S2
Abstract
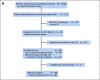
Purpose: NCI-MATCH is a precision medicine trial using genomic testing to allocate patients with advanced malignancies to targeted treatment subprotocols. This report combines two subprotocols evaluating trametinib, a MEK1/2 inhibitor, in patients with Neurofibromatosis 1 (NF1[S1] or GNA11/Q [S2]) altered tumors.
Methods: Eligible patients had tumors with deleterious inactivating NF1 or GNA11/Q mutations by the customized Oncomine AmpliSeq panel. Prior MEK inhibitor treatment was excluded. Glioblastomas (GBMs) were permitted, including malignancies associated with germline NF1 mutations (S1 only). Trametinib was administered at 2 mg once daily over 28-day cycles until toxicity or disease progression. Primary end point was objective response rate (ORR). Secondary end points included progression-free survival (PFS) at 6 months, PFS, and overall survival. Exploratory analyses included co-occurring genomic alterations and PTEN loss.
Results: Fifty patients were eligible and started therapy: 46 with NF1 mutations (S1) and four with GNA11 mutations (S2). In the NF1 cohort, nonsense single-nucleotide variants were identified in 29 and frameshift deletions in 17 tumors. All in S2 had nonuveal melanoma and GNA11 Q209L variant. Two partial responses (PR) were noted in S1, one patient each with advanced lung cancer and GBM for an ORR of 4.3% (90% CI, 0.8 to 13.1). One patient with melanoma in S2 had a PR (ORR, 25%; 90% CI, 1.3 to 75.1). Prolonged stable disease (SD) was also noted in five patients (four in S1 and one in S2) with additional rare histologies. Adverse events were as previously described with trametinib. Comutations in TP53 and PIK3CA were common.
Conclusion: Although these subprotocols did not meet the primary end point for ORR, significant responses or prolonged SD noted in some disease subtypes warrants further investigation.
Trial registration: ClinicalTrials.gov NCT02465060.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures





References
-
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. - PubMed
-
- Imperial R, Toor OM, Hussain A, et al. Comprehensive pancancer genomic analysis reveals (RTK)-RAS-RAF-MEK as a key dysregulated pathway in cancer: Its clinical implications. Semin Cancer Biol. 2019;54:14–28. - PubMed
-
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med. 2012;367:107–114. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 CA180868/CA/NCI NIH HHS/United States
- UG1 CA233329/CA/NCI NIH HHS/United States
- UG1 CA233302/CA/NCI NIH HHS/United States
- UG1 CA233180/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA180794/CA/NCI NIH HHS/United States
- UG1 CA233184/CA/NCI NIH HHS/United States
- UG1 CA233328/CA/NCI NIH HHS/United States
- U24 CA196172/CA/NCI NIH HHS/United States
- P30 CA014520/CA/NCI NIH HHS/United States
- UG1 CA189816/CA/NCI NIH HHS/United States
- UG1 CA233341/CA/NCI NIH HHS/United States
- UG1 CA233277/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

