An approach to rapid distributed manufacturing of broad spectrum anti-viral griffithsin using cell-free systems to mitigate pandemics
- PMID: 37054948
- PMCID: PMC10330340
- DOI: 10.1016/j.nbt.2023.04.003
An approach to rapid distributed manufacturing of broad spectrum anti-viral griffithsin using cell-free systems to mitigate pandemics
Abstract
This study describes the cell-free biomanufacturing of a broad-spectrum antiviral protein, griffithsin (GRFT) such that it can be produced in microgram quantities with consistent purity and potency in less than 24 h. We demonstrate GRFT production using two independent cell-free systems, one plant and one microbial. Griffithsin purity and quality were verified using standard regulatory metrics. Efficacy was demonstrated in vitro against SARS-CoV-2 and HIV-1 and was nearly identical to that of GRFT expressed in vivo. The proposed production process is efficient and can be readily scaled up and deployed wherever a viral pathogen might emerge. The current emergence of viral variants of SARS-CoV-2 has resulted in frequent updating of existing vaccines and loss of efficacy for front-line monoclonal antibody therapies. Proteins such as GRFT with its efficacious and broad virus neutralizing capability provide a compelling pandemic mitigation strategy to promptly suppress viral emergence at the source of an outbreak.
Keywords: Antiviral griffithsin; Cell-free protein synthesis (CFPS); Distributed manufacturing; Pandemic preparedness; SARS-CoV-2.
Copyright © 2023. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest GR, BO and JS have several patents related to the topics discussed in the manuscript.
Figures

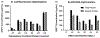
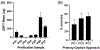

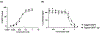
Update of
-
An approach to rapid distributed manufacturing of broad spectrum anti-viral griffithsin using cell-free systems to mitigate pandemics.bioRxiv [Preprint]. 2022 Dec 20:2022.12.19.521044. doi: 10.1101/2022.12.19.521044. bioRxiv. 2022. Update in: N Biotechnol. 2023 Sep 25;76:13-22. doi: 10.1016/j.nbt.2023.04.003. PMID: 36597541 Free PMC article. Updated. Preprint.
Similar articles
-
An approach to rapid distributed manufacturing of broad spectrum anti-viral griffithsin using cell-free systems to mitigate pandemics.bioRxiv [Preprint]. 2022 Dec 20:2022.12.19.521044. doi: 10.1101/2022.12.19.521044. bioRxiv. 2022. Update in: N Biotechnol. 2023 Sep 25;76:13-22. doi: 10.1016/j.nbt.2023.04.003. PMID: 36597541 Free PMC article. Updated. Preprint.
-
Inhibition of influenza A virus and SARS-CoV-2 infection or co-infection by griffithsin and griffithsin-based bivalent entry inhibitor.mBio. 2024 May 8;15(5):e0074124. doi: 10.1128/mbio.00741-24. Epub 2024 Apr 9. mBio. 2024. PMID: 38587427 Free PMC article.
-
Activity of and effect of subcutaneous treatment with the broad-spectrum antiviral lectin griffithsin in two laboratory rodent models.Antimicrob Agents Chemother. 2014;58(1):120-7. doi: 10.1128/AAC.01407-13. Epub 2013 Oct 21. Antimicrob Agents Chemother. 2014. PMID: 24145548 Free PMC article.
-
Broad-Spectrum Anti-coronavirus Vaccines and Therapeutics to Combat the Current COVID-19 Pandemic and Future Coronavirus Disease Outbreaks.Stem Cell Reports. 2021 Mar 9;16(3):398-411. doi: 10.1016/j.stemcr.2020.12.010. Stem Cell Reports. 2021. PMID: 33691145 Free PMC article. Review.
-
Stand Up to Stand Out: Natural Dietary Polyphenols Curcumin, Resveratrol, and Gossypol as Potential Therapeutic Candidates against Severe Acute Respiratory Syndrome Coronavirus 2 Infection.Nutrients. 2023 Sep 6;15(18):3885. doi: 10.3390/nu15183885. Nutrients. 2023. PMID: 37764669 Free PMC article. Review.
Cited by
-
The Antiviral Activity of the Lectin Griffithsin against SARS-CoV-2 Is Enhanced by the Presence of Structural Proteins.Viruses. 2023 Dec 18;15(12):2452. doi: 10.3390/v15122452. Viruses. 2023. PMID: 38140693 Free PMC article.
-
A Cell-Free Protein Synthesis Platform to Produce a Clinically Relevant Allergen Panel.ACS Synth Biol. 2023 Aug 18;12(8):2252-2261. doi: 10.1021/acssynbio.3c00269. Epub 2023 Aug 8. ACS Synth Biol. 2023. PMID: 37553068 Free PMC article.
-
Algal Lectin Griffithsin Inhibits Ebola Virus Infection.Molecules. 2025 Feb 14;30(4):892. doi: 10.3390/molecules30040892. Molecules. 2025. PMID: 40005201 Free PMC article.
-
Isolation and structure elucidation of Dm-CVNH, a new cyanovirin-N homolog with activity against SARS-CoV-2 and HIV-1.J Biol Chem. 2025 Mar;301(3):108319. doi: 10.1016/j.jbc.2025.108319. Epub 2025 Feb 14. J Biol Chem. 2025. PMID: 39956341 Free PMC article.
References
-
- Stratton C, LeFever A. Gmp Facility Requirements in the United States. Hematopoietic Stem Cell Transplantation and Cellular Therapies for Autoimmune Disease, 2021, p. 117. 10.1201/9781315151366-14. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

