Ptf1a expression is necessary for correct targeting of spiral ganglion neurons within the cochlear nuclei
- PMID: 37055006
- PMCID: PMC10210513
- DOI: 10.1016/j.neulet.2023.137244
Ptf1a expression is necessary for correct targeting of spiral ganglion neurons within the cochlear nuclei
Abstract
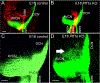
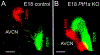
Two transcription factors, Atoh1 and Ptf1a, are essential for cochlear nuclei development. Atoh1 is needed to develop glutamatergic neurons, while Ptf1a is required to generate glycinergic and GABAergic neurons that migrate into the cochlear nucleus. While central projections of inner ear afferents are normal following loss of Atoh1, we wanted to know whether the loss of Ptf1a affects central projections. We found that in Ptf1a mutants, initially, afferents show a normal projection; however, a transient posterior expansion of projections to the dorsal cochlear nucleus occurs at a later stage. In addition, in older (E18.5) Ptf1a mutant mice, excessive neuronal branches form beyond the normal projection to the anterior and posterior ventral cochlear nuclei. Our results on Ptf1a null mice are comparable to that observed in loss of function Prickel1, Npr2, or Fzd3 mouse mutants. The disorganized tonotopic projections that we report in Ptf1a mutant embryos might be functionally relevant, but testing this hypothesis requires Ptf1a KO mice at postnatal stages that unfortunately cannot be performed due to their early death.
Keywords: Auditory system; Cochlear projections; Ear central projections; Hindbrain; Ptf1a.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Npr2 null mutants show initial overshooting followed by reduction of spiral ganglion axon projections combined with near-normal cochleotopic projection.Cell Tissue Res. 2019 Oct;378(1):15-32. doi: 10.1007/s00441-019-03050-6. Epub 2019 Jun 14. Cell Tissue Res. 2019. PMID: 31201541 Free PMC article.
-
ISL1 is necessary for auditory neuron development and contributes toward tonotopic organization.Proc Natl Acad Sci U S A. 2022 Sep 13;119(37):e2207433119. doi: 10.1073/pnas.2207433119. Epub 2022 Sep 8. Proc Natl Acad Sci U S A. 2022. PMID: 36074819 Free PMC article.
-
Lmx1a and Lmx1b are Redundantly Required for the Development of Multiple Components of the Mammalian Auditory System.Neuroscience. 2021 Jan 1;452:247-264. doi: 10.1016/j.neuroscience.2020.11.013. Epub 2020 Nov 24. Neuroscience. 2021. PMID: 33246067 Free PMC article.
-
Development in the Mammalian Auditory System Depends on Transcription Factors.Int J Mol Sci. 2021 Apr 18;22(8):4189. doi: 10.3390/ijms22084189. Int J Mol Sci. 2021. PMID: 33919542 Free PMC article. Review.
-
Age-Related Hearing Loss: Sensory and Neural Etiology and Their Interdependence.Front Aging Neurosci. 2022 Feb 17;14:814528. doi: 10.3389/fnagi.2022.814528. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35250542 Free PMC article. Review.
Cited by
-
Irx3/5 Null Deletion in Mice Blocks Cochlea-Saccule Segregation and Disrupts the Auditory Tonotopic Map.J Comp Neurol. 2024 Dec;532(12):e70008. doi: 10.1002/cne.70008. J Comp Neurol. 2024. PMID: 39655644 Free PMC article.
-
Lmx1a is essential for marginal cell differentiation and stria vascularis formation.Front Cell Dev Biol. 2025 Mar 5;13:1537505. doi: 10.3389/fcell.2025.1537505. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40109362 Free PMC article.
-
Harmony in the Molecular Orchestra of Hearing: Developmental Mechanisms from the Ear to the Brain.Annu Rev Neurosci. 2024 Aug;47(1):1-20. doi: 10.1146/annurev-neuro-081423-093942. Epub 2024 Jul 1. Annu Rev Neurosci. 2024. PMID: 38360566 Free PMC article. Review.
-
Abnormal outer hair cell efferent innervation in Hoxb1-dependent sensorineural hearing loss.PLoS Genet. 2023 Sep 22;19(9):e1010933. doi: 10.1371/journal.pgen.1010933. eCollection 2023 Sep. PLoS Genet. 2023. PMID: 37738262 Free PMC article.
-
Molecular Cascades That Build and Connect Auditory Neurons from Hair Cells to the Auditory Cortex.J Exp Neurol. 2025;6(3):111-120. J Exp Neurol. 2025. PMID: 40823538 Free PMC article.
References
-
- Duncan JS, Elliott KL, Kersigo J, Gray B, Fritzsch B, 2015. Combining whole-mount in situ hybridization with neuronal tracing and immunohistochemistry, In Situ Hybridization Methods. Springer, pp. 339–352.
-
- Duncan JS, Sheltz-Kempf SN, Elliott KL, 2022. Morphological and Molecular Ontogeny of the Auditory System, Evolution of Neurosensory Cells and Systems. CRC Press, pp. 175–200.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

