The life and times of a tRNA
- PMID: 37055150
- PMCID: PMC10275265
- DOI: 10.1261/rna.079620.123
The life and times of a tRNA
Abstract
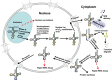
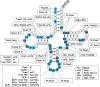
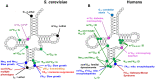
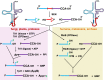
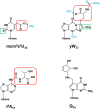
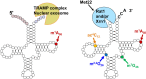
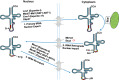
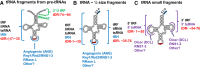
The study of eukaryotic tRNA processing has given rise to an explosion of new information and insights in the last several years. We now have unprecedented knowledge of each step in the tRNA processing pathway, revealing unexpected twists in biochemical pathways, multiple new connections with regulatory pathways, and numerous biological effects of defects in processing steps that have profound consequences throughout eukaryotes, leading to growth phenotypes in the yeast Saccharomyces cerevisiae and to neurological and other disorders in humans. This review highlights seminal new results within the pathways that comprise the life of a tRNA, from its birth after transcription until its death by decay. We focus on new findings and revelations in each step of the pathway including the end-processing and splicing steps, many of the numerous modifications throughout the main body and anticodon loop of tRNA that are so crucial for tRNA function, the intricate tRNA trafficking pathways, and the quality control decay pathways, as well as the biogenesis and biology of tRNA-derived fragments. We also describe the many interactions of these pathways with signaling and other pathways in the cell.
Keywords: decay; modification; splicing; tRNA; tRNA-derived fragments.
© 2023 Phizicky and Hopper; Published by Cold Spring Harbor Laboratory Press for the RNA Society.
Figures

References
-
- Abdel-Fattah W, Jablonowski D, Di Santo R, Thuring KL, Scheidt V, Hammermeister A, Ten Have S, Helm M, Schaffrath R, Stark MJ. 2015. Phosphorylation of Elp1 by Hrr25 is required for elongator-dependent tRNA modification in yeast. PLoS Genet 11: e1004931. 10.1371/journal.pgen.1004931 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
