VGLUT3 Deletion Rescues Motor Deficits and Neuronal Loss in the zQ175 Mouse Model of Huntington's Disease
- PMID: 37055181
- PMCID: PMC10255028
- DOI: 10.1523/JNEUROSCI.0014-23.2023
VGLUT3 Deletion Rescues Motor Deficits and Neuronal Loss in the zQ175 Mouse Model of Huntington's Disease
Abstract
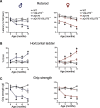
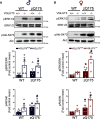
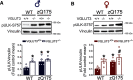
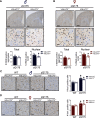
Huntington's disease (HD) is an autosomal-dominant neurodegenerative disease characterized by progressive motor and cognitive impairments, with no disease-modifying therapies yet available. HD pathophysiology involves evident impairment in glutamatergic neurotransmission leading to severe striatal neurodegeneration. The vesicular glutamate transporter-3 (VGLUT3) regulates the striatal network that is centrally affected by HD. Nevertheless, current evidence on the role of VGLUT3 in HD pathophysiology is lacking. Here, we crossed mice lacking Slc17a8 gene (VGLUT3 -/-) with heterozygous zQ175 knock-in mouse model of HD (zQ175:VGLUT3 -/-). Longitudinal assessment of motor and cognitive functions from 6 to 15 months of age reveals that VGLUT3 deletion rescues motor coordination and short-term memory deficits in both male and female zQ175 mice. VGLUT3 deletion also rescues neuronal loss likely via the activation of Akt and ERK1/2 in the striatum of zQ175 mice of both sexes. Interestingly, the rescue in neuronal survival in zQ175:VGLUT3 -/- mice is accompanied by a reduction in the number of nuclear mutant huntingtin (mHTT) aggregates with no change in the total aggregate levels or microgliosis. Collectively, these findings provide novel evidence that VGLUT3, despite its limited expression, can be a vital contributor to HD pathophysiology and a viable target for HD therapeutics.SIGNIFICANCE STATEMENT Dysregulation of the striatal network centrally contributes to the pathophysiology of Huntington's disease (HD). The atypical vesicular glutamate transporter-3 (VGLUT3) has been shown to regulate several major striatal pathologies, such as addiction, eating disorders, or L-DOPA-induced dyskinesia. Yet, our understanding of VGLUT3's role in HD remains unclear. We report here that deletion of the Slc17a8 (Vglut3) gene rescues the deficits in both motor and cognitive functions in HD mice of both sexes. We also find that VGLUT3 deletion activates neuronal survival signaling and reduces nuclear aggregation of abnormal huntingtin proteins and striatal neuron loss in HD mice. Our novel findings highlight the vital contribution of VGLUT3 in HD pathophysiology that can be exploited for HD therapeutic management.
Keywords: Huntington's disease; glutamate; motor dysfunction; neurodegeneration, microglia.
Copyright © 2023 the authors.
Figures



Similar articles
-
Metabotropic Glutamate Receptor 2/3 Activation Improves Motor Performance and Reduces Pathology in Heterozygous zQ175 Huntington Disease Mice.J Pharmacol Exp Ther. 2021 Oct;379(1):74-84. doi: 10.1124/jpet.121.000735. Epub 2021 Jul 30. J Pharmacol Exp Ther. 2021. PMID: 34330748
-
Selective reduction of striatal mature BDNF without induction of proBDNF in the zQ175 mouse model of Huntington's disease.Neurobiol Dis. 2015 Oct;82:466-477. doi: 10.1016/j.nbd.2015.08.008. Epub 2015 Aug 15. Neurobiol Dis. 2015. PMID: 26282324 Free PMC article.
-
Absence of hippocampal pathology persists in the Q175DN mouse model of Huntington's disease despite elevated HTT aggregation.J Huntingtons Dis. 2025 Feb;14(1):59-84. doi: 10.1177/18796397251316762. Epub 2025 Feb 3. J Huntingtons Dis. 2025. PMID: 39973391 Free PMC article.
-
Striatal Induction and Spread of the Huntington's Disease Protein: A Novel Rhes Route.J Huntingtons Dis. 2022;11(3):281-290. doi: 10.3233/JHD-220548. J Huntingtons Dis. 2022. PMID: 35871361 Free PMC article. Review.
-
Implications of Tau Dysregulation in Huntington's Disease and Potential for New Therapeutics.J Huntingtons Dis. 2023;12(1):1-13. doi: 10.3233/JHD-230569. J Huntingtons Dis. 2023. PMID: 37092231 Free PMC article. Review.
Cited by
-
Peripheral sequestration of huntingtin delays neuronal death and depends on N-terminal ubiquitination.Commun Biol. 2024 Aug 18;7(1):1014. doi: 10.1038/s42003-024-06733-1. Commun Biol. 2024. PMID: 39155290 Free PMC article.
References
-
- Abd-Elrahman KS, Albaker A, de Souza JM, Ribeiro FM, Schlossmacher MG, Tiberi M, Hamilton A, Ferguson SS (2020) Aβ oligomers induce pathophysiological mGluR5 signaling in Alzheimer's disease model mice in a sex-selective manner. Sci Signal 13:eabd2494. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
