Long-Term Outcomes in IgA Nephropathy
- PMID: 37055195
- PMCID: PMC10278810
- DOI: 10.2215/CJN.0000000000000135
Long-Term Outcomes in IgA Nephropathy
Abstract
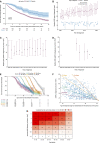
Background: IgA nephropathy can progress to kidney failure, and risk assessment soon after diagnosis has advantages both for clinical management and the development of new therapeutics. We present relationships among proteinuria, eGFR slope, and lifetime risks for kidney failure.
Methods: The IgA nephropathy cohort (2299 adults and 140 children) of the UK National Registry of Rare Kidney Diseases (RaDaR) was analyzed. Patients enrolled had a biopsy-proven diagnosis of IgA nephropathy plus proteinuria >0.5 g/d or eGFR <60 ml/min per 1.73 m 2 . Incident and prevalent populations and a population representative of a typical phase 3 clinical trial cohort were studied. Analyses of kidney survival were conducted using Kaplan-Meier and Cox regression. eGFR slope was estimated using linear mixed models with random intercept and slope.
Results: The median (Q1, Q3) follow-up was 5.9 (3.0, 10.5) years; 50% of patients reached kidney failure or died in the study period. The median (95% confidence interval [CI]) kidney survival was 11.4 (10.5 to 12.5) years; the mean age at kidney failure/death was 48 years, and most patients progressed to kidney failure within 10-15 years. On the basis of eGFR and age at diagnosis, almost all patients were at risk of progression to kidney failure within their expected lifetime unless a rate of eGFR loss ≤1 ml/min per 1.73 m 2 per year was maintained. Time-averaged proteinuria was significantly associated with worse kidney survival and more rapid eGFR loss in incident, prevalent, and clinical trial populations. Thirty percent of patients with time-averaged proteinuria of 0.44 to <0.88 g/g and approximately 20% of patients with time-averaged proteinuria <0.44 g/g developed kidney failure within 10 years. In the clinical trial population, each 10% decrease in time-averaged proteinuria from baseline was associated with a hazard ratio (95% CI) for kidney failure/death of 0.89 (0.87 to 0.92).
Conclusions: Outcomes in this large IgA nephropathy cohort are generally poor with few patients expected to avoid kidney failure in their lifetime. Significantly, patients traditionally regarded as being low risk, with proteinuria <0.88 g/g (<100 mg/mmol), had high rates of kidney failure within 10 years.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Society of Nephrology.
Conflict of interest statement
J. Barratt reports consultancy for Alnylam Pharmaceuticals, Argenx, Astellas, BioCryst, Calliditas, Chinook Therapeutics, Dimerix, Galapagos, GSK, Novartis, Omeros, Travere Therapeutics, UCB, Vera Therapeutics, and Visterra; research funding from Argenx, Calliditas, Chinook, Galapagos, GlaxoSmithKline, Novartis, Omeros, Travere Therapeutics, and Visterra; and advisory or leadership roles on the Editorial Boards of
Figures




Comment in
-
Prognosis of IgA Nephropathy: A Lifetime Story.Clin J Am Soc Nephrol. 2023 Jun 1;18(6):699-701. doi: 10.2215/CJN.0000000000000171. Epub 2023 May 15. Clin J Am Soc Nephrol. 2023. PMID: 37186555 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

