Exercise during CHemotherapy for Ovarian cancer (ECHO) trial: design and implementation of a randomised controlled trial
- PMID: 37055210
- PMCID: PMC10106078
- DOI: 10.1136/bmjopen-2022-067925
Exercise during CHemotherapy for Ovarian cancer (ECHO) trial: design and implementation of a randomised controlled trial
Abstract
Introduction:
Epidemiological evidence supports an association between higher levels of physical activity and improved cancer survival. Trial evidence is now needed to demonstrate the effect of exercise in a clinical setting. The
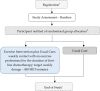
Methods and analysis: Participants (target sample size: n=500) include women with newly diagnosed primary ovarian cancer, scheduled to receive first-line chemotherapy. Consenting participants are randomly allocated (1:1) to either the exercise intervention (plus usual care) or usual care alone, with stratification for recruitment site, age, stage of disease and chemotherapy delivery (neoadjuvant vs adjuvant). The exercise intervention involves individualised exercise prescription with a weekly target of 150 minutes of moderate-intensity, mixed-mode exercise (equivalent to 450 metabolic equivalent minutes per week), delivered for the duration of first-line chemotherapy through weekly telephone sessions with a trial-trained exercise professional. The primary outcomes are progression-free survival and physical well-being. Secondary outcomes include overall survival, physical function, body composition, quality of life, fatigue, sleep, lymphoedema, anxiety, depression, chemotherapy completion rate, chemotherapy-related adverse events, physical activity levels and healthcare usage.
Ethics and dissemination: Ethics approval for the ECHO trial (2019/ETH08923) was granted by the Sydney Local Health District Ethics Review Committee (Royal Prince Alfred Zone) on 21 November 2014. Subsequent approvals were granted for an additional 11 sites across Queensland, New South Wales, Victoria and the Australian Capital Territory. Findings from the ECHO trial are planned to be disseminated via peer-reviewed publications and international exercise and oncology conferences.
Trial registration number: Australian New Zealand Clinical Trial Registry (ANZCTRN12614001311640; https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=367123&isReview=true).
Keywords: GYNAECOLOGY; ONCOLOGY; PUBLIC HEALTH.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
