Excessive serine from the bone marrow microenvironment impairs megakaryopoiesis and thrombopoiesis in Multiple Myeloma
- PMID: 37055385
- PMCID: PMC10102122
- DOI: 10.1038/s41467-023-37699-z
Excessive serine from the bone marrow microenvironment impairs megakaryopoiesis and thrombopoiesis in Multiple Myeloma
Abstract
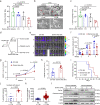
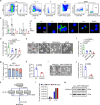
Thrombocytopenia is a major complication in a subset of patients with multiple myeloma (MM). However, little is known about its development and significance during MM. Here, we show thrombocytopenia is linked to poor prognosis in MM. In addition, we identify serine, which is released from MM cells into the bone marrow microenvironment, as a key metabolic factor that suppresses megakaryopoiesis and thrombopoiesis. The impact of excessive serine on thrombocytopenia is mainly mediated through the suppression of megakaryocyte (MK) differentiation. Extrinsic serine is transported into MKs through SLC38A1 and downregulates SVIL via SAM-mediated tri-methylation of H3K9, ultimately leading to the impairment of megakaryopoiesis. Inhibition of serine utilization or treatment with TPO enhances megakaryopoiesis and thrombopoiesis and suppresses MM progression. Together, we identify serine as a key metabolic regulator of thrombocytopenia, unveil molecular mechanisms governing MM progression, and provide potential therapeutic strategies for treating MM patients by targeting thrombocytopenia.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

