IDH3γ functions as a redox switch regulating mitochondrial energy metabolism and contractility in the heart
- PMID: 37055412
- PMCID: PMC10102218
- DOI: 10.1038/s41467-023-37744-x
IDH3γ functions as a redox switch regulating mitochondrial energy metabolism and contractility in the heart
Abstract
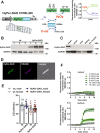
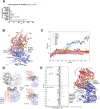
Redox signaling and cardiac function are tightly linked. However, it is largely unknown which protein targets are affected by hydrogen peroxide (H2O2) in cardiomyocytes that underly impaired inotropic effects during oxidative stress. Here, we combine a chemogenetic mouse model (HyPer-DAO mice) and a redox-proteomics approach to identify redox sensitive proteins. Using the HyPer-DAO mice, we demonstrate that increased endogenous production of H2O2 in cardiomyocytes leads to a reversible impairment of cardiac contractility in vivo. Notably, we identify the γ-subunit of the TCA cycle enzyme isocitrate dehydrogenase (IDH)3 as a redox switch, linking its modification to altered mitochondrial metabolism. Using microsecond molecular dynamics simulations and experiments using cysteine-gene-edited cells reveal that IDH3γ Cys148 and 284 are critically involved in the H2O2-dependent regulation of IDH3 activity. Our findings provide an unexpected mechanism by which mitochondrial metabolism can be modulated through redox signaling processes.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Ryter SW, et al. Mechanisms of cell death in oxidative stress. Antioxid. Redox Signal. 2007;9:49–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

