Non-functional ubiquitin C-terminal hydrolase L1 drives podocyte injury through impairing proteasomes in autoimmune glomerulonephritis
- PMID: 37055432
- PMCID: PMC10102022
- DOI: 10.1038/s41467-023-37836-8
Non-functional ubiquitin C-terminal hydrolase L1 drives podocyte injury through impairing proteasomes in autoimmune glomerulonephritis
Erratum in
-
Publisher Correction: Non-functional ubiquitin C-terminal hydrolase L1 drives podocyte injury through impairing proteasomes in autoimmune glomerulonephritis.Nat Commun. 2023 Apr 28;14(1):2453. doi: 10.1038/s41467-023-38206-0. Nat Commun. 2023. PMID: 37117182 Free PMC article. No abstract available.
Abstract
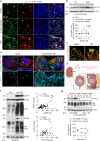
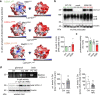
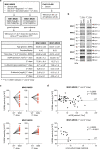
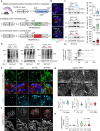
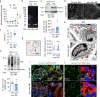
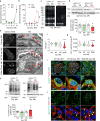
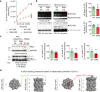
Little is known about the mechanistic significance of the ubiquitin proteasome system (UPS) in a kidney autoimmune environment. In membranous nephropathy (MN), autoantibodies target podocytes of the glomerular filter resulting in proteinuria. Converging biochemical, structural, mouse pathomechanistic, and clinical information we report that the deubiquitinase Ubiquitin C-terminal hydrolase L1 (UCH-L1) is induced by oxidative stress in podocytes and is directly involved in proteasome substrate accumulation. Mechanistically, this toxic gain-of-function is mediated by non-functional UCH-L1, which interacts with and thereby impairs proteasomes. In experimental MN, UCH-L1 becomes non-functional and MN patients with poor outcome exhibit autoantibodies with preferential reactivity to non-functional UCH-L1. Podocyte-specific deletion of UCH-L1 protects from experimental MN, whereas overexpression of non-functional UCH-L1 impairs podocyte proteostasis and drives injury in mice. In conclusion, the UPS is pathomechanistically linked to podocyte disease by aberrant proteasomal interactions of non-functional UCH-L1.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Comment in
-
Dysfunctional UCH-L1 inhibits proteostasis.Nat Rev Nephrol. 2023 Jul;19(7):424. doi: 10.1038/s41581-023-00730-z. Nat Rev Nephrol. 2023. PMID: 37258710 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

