Enriched binocular experience followed by sleep optimally restores binocular visual cortical responses in a mouse model of amblyopia
- PMID: 37055505
- PMCID: PMC10102075
- DOI: 10.1038/s42003-023-04798-y
Enriched binocular experience followed by sleep optimally restores binocular visual cortical responses in a mouse model of amblyopia
Abstract
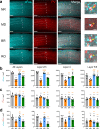
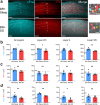
Studies of primary visual cortex have furthered our understanding of amblyopia, long-lasting visual impairment caused by imbalanced input from the two eyes during childhood, which is commonly treated by patching the dominant eye. However, the relative impacts of monocular vs. binocular visual experiences on recovery from amblyopia are unclear. Moreover, while sleep promotes visual cortex plasticity following loss of input from one eye, its role in recovering binocular visual function is unknown. Using monocular deprivation in juvenile male mice to model amblyopia, we compared recovery of cortical neurons' visual responses after identical-duration, identical-quality binocular or monocular visual experiences. We demonstrate that binocular experience is quantitatively superior in restoring binocular responses in visual cortex neurons. However, this recovery was seen only in freely-sleeping mice; post-experience sleep deprivation prevented functional recovery. Thus, both binocular visual experience and subsequent sleep help to optimally renormalize bV1 responses in a mouse model of amblyopia.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Long-term Monocular Deprivation during Juvenile Critical Period Disrupts Binocular Integration in Mouse Visual Thalamus.J Neurosci. 2020 Jan 15;40(3):585-604. doi: 10.1523/JNEUROSCI.1626-19.2019. Epub 2019 Nov 25. J Neurosci. 2020. PMID: 31767678 Free PMC article.
-
Binocular eyelid closure promotes anatomical but not behavioral recovery from monocular deprivation.Vision Res. 2015 Sep;114:151-60. doi: 10.1016/j.visres.2014.12.012. Epub 2014 Dec 20. Vision Res. 2015. PMID: 25536470
-
A special role for binocular visual input during development and as a component of occlusion therapy for treatment of amblyopia.Restor Neurol Neurosci. 2008;26(4-5):425-34. Restor Neurol Neurosci. 2008. PMID: 18997317
-
The case from animal studies for balanced binocular treatment strategies for human amblyopia.Ophthalmic Physiol Opt. 2014 Mar;34(2):129-45. doi: 10.1111/opo.12122. Ophthalmic Physiol Opt. 2014. PMID: 24588531 Review.
-
Harnessing brain plasticity to improve binocular vision in amblyopia: An evidence-based update.Eur J Ophthalmol. 2024 Jul;34(4):901-912. doi: 10.1177/11206721231187426. Epub 2023 Jul 10. Eur J Ophthalmol. 2024. PMID: 37431104 Free PMC article. Review.
Cited by
-
From Synaptic Plasticity to Neurodegeneration: BDNF as a Transformative Target in Medicine.Int J Mol Sci. 2025 Apr 30;26(9):4271. doi: 10.3390/ijms26094271. Int J Mol Sci. 2025. PMID: 40362507 Free PMC article. Review.
-
Early monocular deprivation reduces the capacity for neural plasticity in the cat visual system.Cereb Cortex Commun. 2023 Aug 17;4(3):tgad017. doi: 10.1093/texcom/tgad017. eCollection 2023. Cereb Cortex Commun. 2023. PMID: 37675436 Free PMC article.
-
Brief sleep disruption alters synaptic structures among hippocampal and neocortical somatostatin-expressing interneurons.bioRxiv [Preprint]. 2025 Feb 5:2024.07.22.604591. doi: 10.1101/2024.07.22.604591. bioRxiv. 2025. Update in: Sleep. 2025 Jun 13;48(6):zsaf064. doi: 10.1093/sleep/zsaf064. PMID: 39211205 Free PMC article. Updated. Preprint.
-
Brief sleep disruption alters synaptic structures among hippocampal and neocortical somatostatin-expressing interneurons.Sleep. 2025 Jun 13;48(6):zsaf064. doi: 10.1093/sleep/zsaf064. Sleep. 2025. PMID: 40096531
-
Leveraging neural plasticity for the treatment of amblyopia.Surv Ophthalmol. 2024 Sep-Oct;69(5):818-832. doi: 10.1016/j.survophthal.2024.04.006. Epub 2024 May 18. Surv Ophthalmol. 2024. PMID: 38763223 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

