Predictive biomarkers of immunotherapy response with pharmacological applications in solid tumors
- PMID: 37055532
- PMCID: PMC10462766
- DOI: 10.1038/s41401-023-01079-6
Predictive biomarkers of immunotherapy response with pharmacological applications in solid tumors
Abstract
Immune-checkpoint inhibitors show promising effects in the treatment of multiple tumor types. Biomarkers are biological indicators used to select patients for a systemic anticancer treatment, but there are only a few clinically useful biomarkers such as PD-L1 expression and tumor mutational burden, which can be used to predict immunotherapy response. In this study, we established a database consisting of both gene expression and clinical data to identify biomarkers of response to anti-PD-1, anti-PD-L1, and anti-CTLA-4 immunotherapies. A GEO screening was executed to identify datasets with simultaneously available clinical response and transcriptomic data regardless of cancer type. The screening was restricted to the studies involving administration of anti-PD-1 (nivolumab, pembrolizumab), anti-PD-L1 (atezolizumab, durvalumab) or anti-CTLA-4 (ipilimumab) agents. Receiver operating characteristic (ROC) analysis and Mann-Whitney test were executed across all genes to identify features related to therapy response. The database consisted of 1434 tumor tissue samples from 19 datasets with esophageal, gastric, head and neck, lung, and urothelial cancers, plus melanoma. The strongest druggable gene candidates linked to anti-PD-1 resistance were SPIN1 (AUC = 0.682, P = 9.1E-12), SRC (AUC = 0.667, P = 5.9E-10), SETD7 (AUC = 0.663, P = 1.0E-09), FGFR3 (AUC = 0.657, P = 3.7E-09), YAP1 (AUC = 0.655, P = 6.0E-09), TEAD3 (AUC = 0.649, P = 4.1E-08) and BCL2 (AUC = 0.634, P = 9.7E-08). In the anti-CTLA-4 treatment cohort, BLCAP (AUC = 0.735, P = 2.1E-06) was the most promising gene candidate. No therapeutically relevant target was found to be predictive in the anti-PD-L1 cohort. In the anti-PD-1 group, we were able to confirm the significant correlation with survival for the mismatch-repair genes MLH1 and MSH6. A web platform for further analysis and validation of new biomarker candidates was set up and available at https://www.rocplot.com/immune . In summary, a database and a web platform were established to investigate biomarkers of immunotherapy response in a large cohort of solid tumor samples. Our results could help to identify new patient cohorts eligible for immunotherapy.
Keywords: ROC curve; drug resistance; druggable genes; gene expression; immune checkpoint inhibitors; immunotherapy.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
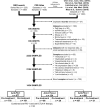
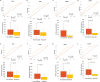

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

