Phytochemistry and pharmacology of natural prenylated flavonoids
- PMID: 37055613
- PMCID: PMC10101826
- DOI: 10.1007/s12272-023-01443-4
Phytochemistry and pharmacology of natural prenylated flavonoids
Abstract
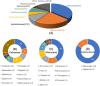
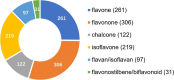
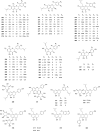
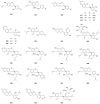
Prenylated flavonoids are a special kind of flavonoid derivative possessing one or more prenyl groups in the parent nucleus of the flavonoid. The presence of the prenyl side chain enriched the structural diversity of flavonoids and increased their bioactivity and bioavailability. Prenylated flavonoids show a wide range of biological activities, such as anti-cancer, anti-inflammatory, neuroprotective, anti-diabetic, anti-obesity, cardioprotective effects, and anti-osteoclastogenic activities. In recent years, many compounds with significant activity have been discovered with the continuous excavation of the medicinal value of prenylated flavonoids, and have attracted the extensive attention of pharmacologists. This review summarizes recent progress on research into natural active prenylated flavonoids to promote new discoveries of their medicinal value.
Keywords: Anti-cancer; Anti-inflammatory; Pharmacological activity; Phytochemistry; Prenylated flavonoid.
© 2023. The Pharmaceutical Society of Korea.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures





























References
-
- Abd-Ellatef GEF, Gazzano E, El-Desoky AH, Hamed AR, Kopecka J, Belisario DC, Costamagna C, Marie S, Fahmy MA, Abdel-Hamid SR, Riganti AZ. Glabratephrin reverses doxorubicin resistance in triple negative breast cancer by inhibiting P-glycoprotein. Pharmacol Res. 2022;175:105975. doi: 10.1016/j.phrs.2021.105975. - DOI - PubMed
-
- Abdullah I, Phongpaichit S, Voravuthikunchai SP, Mahabusarakam W. Prenylated biflavonoids from the green branches of Garcinia dulcis. Phytochem Lett. 2018;23:176–179. doi: 10.1016/j.phytol.2017.12.004. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

