The impact of urine collection method on canine urinary microbiota detection: a cross-sectional study
- PMID: 37055748
- PMCID: PMC10100081
- DOI: 10.1186/s12866-023-02815-y
The impact of urine collection method on canine urinary microbiota detection: a cross-sectional study
Abstract
Background: The urinary tract harbors unique microbial communities that play important roles in urogenital health and disease. Dogs naturally suffer from several of the same urological disorders as humans (e.g., urinary tract infections, neoplasia, urolithiasis) and represent a valuable translational model for studying the role of urinary microbiota in various disease states. Urine collection technique represents a critical component of urinary microbiota research study design. However, the impact of collection method on the characterization of the canine urinary microbiota remains unknown. Therefore, the objective of this study was to determine whether urine collection technique alters the microbial populations detected in canine urine samples. Urine was collected from asymptomatic dogs by both cystocentesis and midstream voiding. Microbial DNA was isolated from each sample and submitted for amplicon sequencing of the V4 region of the bacterial 16 S rRNA gene, followed by analyses to compare microbial diversity and composition between urine collection techniques.
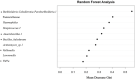
Results: Samples collected via midstream voiding exhibited significantly higher sequence read counts (P = .036) and observed richness (P = .0024) than cystocentesis urine. Bray Curtis and Unweighted UniFrac measures of beta diversity showed distinct differences in microbial composition by collection method (P = .0050, R2 = 0.06 and P = .010, R2 = 0.07, respectively). Seven taxa were identified as differentially abundant between groups. Pasteurellaceae, Haemophilus, Friedmanniella, two variants of Streptococcus, and Fusobacterium were over-represented in voided urine, while a greater abundance of Burkholderia-Caballeronia-Paraburkholderia characterized cystocentesis samples. Analyses were performed at five thresholds for minimum sequence depth and using three data normalization strategies to validate results; patterns of alpha and beta diversity remained consistent regardless of minimum read count requirements or normalization method.
Conclusion: Microbial composition differs in canine urine samples collected via cystocentesis as compared to those collected via midstream voiding. Future researchers should select a single urine collection method based on the biological question of interest when designing canine urinary microbiota studies. Additionally, the authors suggest caution when interpreting results across studies that did not utilize identical urine collection methods.
Keywords: Canine; Cystocentesis; Midstream voiding; Urinary microbiota; Urine collection technique.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Fouts DE, Pieper R, Szpakowski S, Pohl H, Knoblach S, Suh MJ, et al. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J Transl Med. 2012;10:174. doi: 10.1186/1479-5876-10-174. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

