Stalled oligodendrocyte differentiation in IDH-mutant gliomas
- PMID: 37055795
- PMCID: PMC10103394
- DOI: 10.1186/s13073-023-01175-6
Stalled oligodendrocyte differentiation in IDH-mutant gliomas
Abstract
Background: Roughly 50% of adult gliomas harbor isocitrate dehydrogenase (IDH) mutations. According to the 2021 WHO classification guideline, these gliomas are diagnosed as astrocytomas, harboring no 1p19q co-deletion, or oligodendrogliomas, harboring 1p19q co-deletion. Recent studies report that IDH-mutant gliomas share a common developmental hierarchy. However, the neural lineages and differentiation stages in IDH-mutant gliomas remain inadequately characterized.
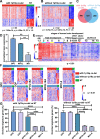
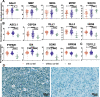
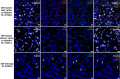
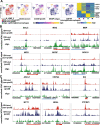
Methods: Using bulk transcriptomes and single-cell transcriptomes, we identified genes enriched in IDH-mutant gliomas with or without 1p19q co-deletion, we also assessed the expression pattern of stage-specific signatures and key regulators of oligodendrocyte lineage differentiation. We compared the expression of oligodendrocyte lineage stage-specific markers between quiescent and proliferating malignant single cells. The gene expression profiles were validated using RNAscope analysis and myelin staining and were further substantiated using data of DNA methylation and single-cell ATAC-seq. As a control, we assessed the expression pattern of astrocyte lineage markers.
Results: Genes concordantly enriched in both subtypes of IDH-mutant gliomas are upregulated in oligodendrocyte progenitor cells (OPC). Signatures of early stages of oligodendrocyte lineage and key regulators of OPC specification and maintenance are enriched in all IDH-mutant gliomas. In contrast, signature of myelin-forming oligodendrocytes, myelination regulators, and myelin components are significantly down-regulated or absent in IDH-mutant gliomas. Further, single-cell transcriptomes of IDH-mutant gliomas are similar to OPC and differentiation-committed oligodendrocyte progenitors, but not to myelinating oligodendrocyte. Most IDH-mutant glioma cells are quiescent; quiescent cells and proliferating cells resemble the same differentiation stage of oligodendrocyte lineage. Mirroring the gene expression profiles along the oligodendrocyte lineage, analyses of DNA methylation and single-cell ATAC-seq data demonstrate that genes of myelination regulators and myelin components are hypermethylated and show inaccessible chromatin status, whereas regulators of OPC specification and maintenance are hypomethylated and show open chromatin status. Markers of astrocyte precursors are not enriched in IDH-mutant gliomas.
Conclusions: Our studies show that despite differences in clinical manifestation and genomic alterations, all IDH-mutant gliomas resemble early stages of oligodendrocyte lineage and are stalled in oligodendrocyte differentiation due to blocked myelination program. These findings provide a framework to accommodate biological features and therapy development for IDH-mutant gliomas.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
-
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

