Intravitreal bevacizumab plus propranolol for neovascular age-related macular degeneration (the BEVALOL study): a phase I clinical trial
- PMID: 37055868
- PMCID: PMC10099020
- DOI: 10.1186/s40942-023-00460-1
Intravitreal bevacizumab plus propranolol for neovascular age-related macular degeneration (the BEVALOL study): a phase I clinical trial
Abstract
Background: Given the persistently large public health impact of neovascular age-related macular degeneration (nARMD) despite many years of anti-VEGF therapy as the first-line treatment and the demonstrated ability of b-blockers to reduce neovascularization, a synergistic effect between an anti-VEGF agent and an intravitreal beta-blocker is important to investigate in the quest for therapeutic alternatives that maximize efficacy and/or reduce costs. The main purpose of this study is to investigate the safety of a 0.1 ml intravitreal injection of a combination of bevacizumab (1.25 mg/0.05 ml) and propranolol (50 g/0.05 ml) to treat nARMD.
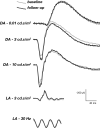
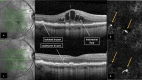
Methods: Prospective phase I clinical trial that included patients with nARMD. Comprehensive ophthalmic evaluation was performed at baseline and included Early Treatment Diabetic Retinopathy Study (ETDRS) best-corrected visual acuity (BCVA), biomicroscopy of the anterior and posterior segments, binocular indirect ophthalmoscopy, color fundus photography, spectral domain optical coherence tomography (OCT), OCT angiography (OCT-A), fluorescein angiography (Spectralis, Heidelberg), and full-field electroretinography (ERG). All eyes were treated with a 0.1 ml intravitreal injection of a combination of bevacizumab (1.25 mg/0.05 ml) and propranolol (50 g/0.05 ml) within 1 week of baseline evaluation. The patients were reexamined at weeks 4, 8 and 12, and clinical evaluation and SD-OCT were performed at all follow-up visits. Additional injections of combination bevacizumab (1.25 mg/0.05 ml) and propranolol (50 g/0.05 ml) were administered at weeks 4 and 8. At the final study evaluation (week 12), color fundus photography, OCT-A, fluorescein angiography, and full-field ERG were repeated.
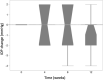
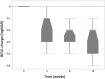
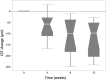
Results: Eleven patients (11 eyes) completed all study visits of the 12 week study. Full field ERG b-waves did not show significant (p < 0.05) changes at week 12 compared to baseline. During the 12 week follow-up period, none of the study eyes developed intraocular inflammation, endophthalmitis or intraocular pressure elevation more than 4 mmHg over baseline. Mean ± SE BCVA (logMAR) was 0.79 ± 0.09 at baseline and was significantly (p < 0.05) improved to 0.61 ± 0.10 at week 4; 0.53 ± 0.10 at week 8; and 0.51 ± 0.09 at week 12. Mean ± SE central subfield thickness (CST) (μm) was 462 ± 45 at baseline and was significantly (p < 0.05) lower at 4, 8 and 12 weeks (385 ± 37; 356 ± 29 and 341 ± 24, respectively).
Conclusions: In this 12 week trial of a combination of intravitreal bevacizumab and propranolol for treatment of nARMD, no adverse events or signals of ocular toxicity were observed. Further studies using this combination therapy are warranted. Trial Registration Project registered in Plataforma Brasil with CAAE number 28108920.0.0000.5440 and approved in ethics committee of Clinics Hospital of Ribeirao Preto Medicine School of São Paulo University-Ribeirão Preto, São Paulo, Brazil (appreciation number 3.999.989 gave the approval).
Keywords: Age-related macular degeneration; Bevacizumab; Maculopathy; Propranolol; VEGF.
© 2023. The Author(s).
Conflict of interest statement
Authors JESTN, FVRC, AM, MML, IUS and RJ declare that they have no conflict of interest.
Figures
Similar articles
-
Intravitreal bevacizumab (IVB) versus IVB in combination with pars plana vitrectomy for vitreous hemorrhage secondary to proliferative diabetic retinopathy: a randomized clinical trial.Int J Retina Vitreous. 2021 Apr 26;7(1):35. doi: 10.1186/s40942-021-00296-7. Int J Retina Vitreous. 2021. PMID: 33902755 Free PMC article.
-
Intravitreal bevacizumab (Avastin) in combination with verteporfin photodynamic therapy for choroidal neovascularization associated with age-related macular degeneration (IBeVe Study).Graefes Arch Clin Exp Ophthalmol. 2007 Sep;245(9):1273-80. doi: 10.1007/s00417-007-0557-x. Epub 2007 Feb 28. Graefes Arch Clin Exp Ophthalmol. 2007. PMID: 17333238 Clinical Trial.
-
One-year outcomes of less frequent bevacizumab in age-related macular degeneration.Retina. 2011 Apr;31(4):645-53. doi: 10.1097/IAE.0b013e3182012d18. Retina. 2011. PMID: 21358363
-
Treat-and-extend bevacizumab for neovascular age-related macular degeneration: the importance of baseline characteristics.Retina. 2014 May;34(5):846-52. doi: 10.1097/IAE.0000000000000033. Retina. 2014. PMID: 24240560
-
Treatment of neovascular age-related macular degeneration with anti-vascular endothelial growth factor drugs: progress from mechanisms to clinical applications.Front Med (Lausanne). 2024 Jul 19;11:1411278. doi: 10.3389/fmed.2024.1411278. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39099595 Free PMC article. Review.
Cited by
-
Risk of progression in intermediate age-related macular degeneration among patients using systemic beta-blockers.Front Ophthalmol (Lausanne). 2025 Apr 14;5:1535791. doi: 10.3389/fopht.2025.1535791. eCollection 2025. Front Ophthalmol (Lausanne). 2025. PMID: 40297766 Free PMC article.
-
Recent advances and applications of optical coherence tomography angiography in diabetic retinopathy.Front Endocrinol (Lausanne). 2025 Apr 16;16:1438739. doi: 10.3389/fendo.2025.1438739. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40309445 Free PMC article.
-
A promising case of preclinical-clinical translation: β-adrenoceptor blockade from the oxygen-induced retinopathy model to retinopathy of prematurity.Front Physiol. 2024 Jun 13;15:1408605. doi: 10.3389/fphys.2024.1408605. eCollection 2024. Front Physiol. 2024. PMID: 38938747 Free PMC article. Review.
References
-
- Campochiaro PA, Soloway P, Ryan SJ, Miller JW. The pathogenesis of choroidal neovascularization in patients with age-related macular degeneration. Mol Vis. 1999;5:34. - PubMed
-
- Virgili G, Parravano M, Menchini F, Evans JR. Anti-vascular endothelial growth factor for diabetic macular oedema. Cochrane Database Syst Rev. 2014;10:CD007419. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

