Copper metabolism in cell death and autophagy
- PMID: 37055935
- PMCID: PMC10351475
- DOI: 10.1080/15548627.2023.2200554
Copper metabolism in cell death and autophagy
Abstract
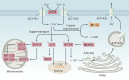
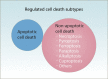
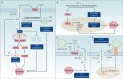
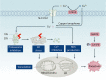
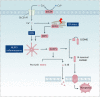
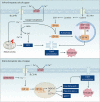
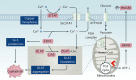
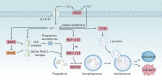
Copper is an essential trace element in biological systems, maintaining the activity of enzymes and the function of transcription factors. However, at high concentrations, copper ions show increased toxicity by inducing regulated cell death, such as apoptosis, paraptosis, pyroptosis, ferroptosis, and cuproptosis. Furthermore, copper ions can trigger macroautophagy/autophagy, a lysosome-dependent degradation pathway that plays a dual role in regulating the survival or death fate of cells under various stress conditions. Pathologically, impaired copper metabolism due to environmental or genetic causes is implicated in a variety of human diseases, such as rare Wilson disease and common cancers. Therapeutically, copper-based compounds are potential chemotherapeutic agents that can be used alone or in combination with other drugs or approaches to treat cancer. Here, we review the progress made in understanding copper metabolic processes and their impact on the regulation of cell death and autophagy. This knowledge may help in the design of future clinical tools to improve cancer diagnosis and treatment.Abbreviations: ACSL4, acyl-CoA synthetase long chain family member 4; AIFM1/AIF, apoptosis inducing factor mitochondria associated 1; AIFM2, apoptosis inducing factor mitochondria associated 2; ALDH, aldehyde dehydrogenase; ALOX, arachidonate lipoxygenase; AMPK, AMP-activated protein kinase; APAF1, apoptotic peptidase activating factor 1; ATF4, activating transcription factor 4; ATG, autophagy related; ATG13, autophagy related 13; ATG5, autophagy related 5; ATOX1, antioxidant 1 copper chaperone; ATP, adenosine triphosphate; ATP7A, ATPase copper transporting alpha; ATP7B, ATPase copper transporting beta; BAK1, BCL2 antagonist/killer 1; BAX, BCL2 associated X apoptosis regulator; BBC3/PUMA, BCL2 binding component 3; BCS, bathocuproinedisulfonic acid; BECN1, beclin 1; BID, BH3 interacting domain death agonist; BRCA1, BRCA1 DNA repair associated; BSO, buthionine sulphoximine; CASP1, caspase 1; CASP3, caspase 3; CASP4/CASP11, caspase 4; CASP5, caspase 5; CASP8, caspase 8; CASP9, caspase 9; CCS, copper chaperone for superoxide dismutase; CD274/PD-L1, CD274 molecule; CDH2, cadherin 2; CDKN1A/p21, cyclin dependent kinase inhibitor 1A; CDKN1B/p27, cyclin-dependent kinase inhibitor 1B; COMMD10, COMM domain containing 10; CoQ10, coenzyme Q 10; CoQ10H2, reduced coenzyme Q 10; COX11, cytochrome c oxidase copper chaperone COX11; COX17, cytochrome c oxidase copper chaperone COX17; CP, ceruloplasmin; CYCS, cytochrome c, somatic; DBH, dopamine beta-hydroxylase; DDIT3/CHOP, DNA damage inducible transcript 3; DLAT, dihydrolipoamide S-acetyltransferase; DTC, diethyldithiocarbamate; EIF2A, eukaryotic translation initiation factor 2A; EIF2AK3/PERK, eukaryotic translation initiation factor 2 alpha kinase 3; ER, endoplasmic reticulum; ESCRT-III, endosomal sorting complex required for transport-III; ETC, electron transport chain; FABP3, fatty acid binding protein 3; FABP7, fatty acid binding protein 7; FADD, Fas associated via death domain; FAS, Fas cell surface death receptor; FASL, Fas ligand; FDX1, ferredoxin 1; GNAQ/11, G protein subunit alpha q/11; GPX4, glutathione peroxidase 4; GSDMD, gasdermin D; GSH, glutathione; HDAC, histone deacetylase; HIF1, hypoxia inducible factor 1; HIF1A, hypoxia inducible factor 1 subunit alpha; HMGB1, high mobility group box 1; IL1B, interleukin 1 beta; IL17, interleukin 17; KRAS, KRAS proto-oncogene, GTPase; LOX, lysyl oxidase; LPCAT3, lysophosphatidylcholine acyltransferase 3; MAP1LC3, microtubule associated protein 1 light chain 3; MAP2K1, mitogen-activated protein kinase kinase 1; MAP2K2, mitogen-activated protein kinase kinase 2; MAPK, mitogen-activated protein kinases; MAPK14/p38, mitogen-activated protein kinase 14; MEMO1, mediator of cell motility 1; MT-CO1/COX1, mitochondrially encoded cytochrome c oxidase I; MT-CO2/COX2, mitochondrially encoded cytochrome c oxidase II; MTOR, mechanistic target of rapamycin kinase; MTs, metallothioneins; NAC, N-acetylcysteine; NFKB/NF-Κb, nuclear factor kappa B; NLRP3, NLR family pyrin domain containing 3; NPLOC4/NPL4, NPL4 homolog ubiquitin recognition factor; PDE3B, phosphodiesterase 3B; PDK1, phosphoinositide dependent protein kinase 1; PHD, prolyl-4-hydroxylase domain; PIK3C3/VPS34, phosphatidylinositol 3-kinase catalytic subunit type 3; PMAIP1/NOXA, phorbol-12-myristate-13-acetate-induced protein 1; POR, cytochrome P450 oxidoreductase; PUFA-PL, PUFA of phospholipids; PUFAs, polyunsaturated fatty acids; ROS, reactive oxygen species; SCO1, synthesis of cytochrome C oxidase 1; SCO2, synthesis of cytochrome C oxidase 2; SLC7A11, solute carrier family 7 member 11; SLC11A2/DMT1, solute carrier family 11 member 2; SLC31A1/CTR1, solute carrier family 31 member 1; SLC47A1, solute carrier family 47 member 1; SOD1, superoxide dismutase; SP1, Sp1 transcription factor; SQSTM1/p62, sequestosome 1; STEAP4, STEAP4 metalloreductase; TAX1BP1, Tax1 binding protein 1; TEPA, tetraethylenepentamine; TFEB, transcription factor EB; TM, tetrathiomolybdate; TP53/p53, tumor protein p53; TXNRD1, thioredoxin reductase 1; UCHL5, ubiquitin C-terminal hydrolase L5; ULK1, Unc-51 like autophagy activating kinase 1; ULK1, unc-51 like autophagy activating kinase 1; ULK2, unc-51 like autophagy activating kinase 2; USP14, ubiquitin specific peptidase 14; VEGF, vascular endothelial gro wth factor; XIAP, X-linked inhibitor of apoptosis.
Keywords: autophagy; cancer; cell death; copper; cuproptosis; ferroptosis.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures

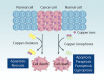
References
-
- Chen J, Jiang Y, Shi H, et al. The molecular mechanisms of copper metabolism and its roles in human diseases. Pflügers Archiv - European Journal of Physiology. 2020;472(10):1415–1429. - PubMed
-
- Steffens GC, Biewald R, Buse G.. Cytochrome c oxidase is a three-copper, two-heme-A protein. Eur J Biochem. 1987;164(2):295–300. - PubMed
-
- In: Harris ED. Copper as acofactor and regulator of copper,zinc superoxide dismutase. J Nutr 1992. Vol. 122pp. 636–640 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
