Cost-effectiveness analysis of 'test and treat' policy for antiretroviral therapy among heterosexual HIV population in India
- PMID: 37056069
- PMCID: PMC10278921
- DOI: 10.4103/ijmr.IJMR_806_20
Cost-effectiveness analysis of 'test and treat' policy for antiretroviral therapy among heterosexual HIV population in India
Abstract
Background & objectives: The World Health Organisation recommended immediate initiation of antiretroviral therapy (ART) in all adult human immunodeficiency virus (HIV) patients regardless of their CD4 cell count. This study was undertaken to ascertain the cost-effectiveness of implementation of these guidelines in India.
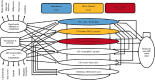
Methods: A Markov model was developed to assess the lifetime costs and health outcomes of three scenarios for initiation of ART treatment at varying CD4 cell count <350/mm[3], <500/mm[3] and test and treat using health system perspective using life-time horizon. A few input parameters for this model namely, transition probabilities from one stage to another stage of HIV and incidence rates of TB were calculated from the data of Centre of Excellence for HIV treatment and care, Chandigarh; whereas, other parameters were obtained from the published literature. Total HIV-related deaths averted, HIV infections averted and incremental cost-effectiveness ratio per quality adjusted life years (QALYs) gained were calculated.
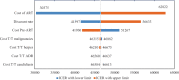
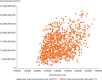
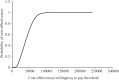
Result: Test and treat intervention slowed down the progression of disease and averted 18,386 HIV-related deaths, over lifetime horizon. It also averted 16,105 new HIV infections and saved 343,172 QALYs as compared to the strategy of starting ART at CD4 cell count of 500/mm[3]. Incremental cost per QALY gained for the immediate initiation of ART as compared to ART at CD4 cell count of 500/mm[3] and 350/mm[3] was ₹ 46,599 and 80,050, respectively at reported rates of adherence to the therapy.
Interpretation & conclusions: Immediate ART (test and treat) is highly cost-effective strategy over the past criteria of delayed therapy in India. Cost-effectiveness of this policy is largely because of reduction in the transmission of HIV.
Keywords: Antiretroviral therapy; CD4; HIV; India –; cost-effectiveness analyses; economic evaluation; modelling; test and treat.
Conflict of interest statement
Figures
Comment on
-
Economic and epidemiological impact of early antiretroviral therapy initiation in India.J Int AIDS Soc. 2015 Oct 1;18(1):20217. doi: 10.7448/IAS.18.1.20217. eCollection 2015. J Int AIDS Soc. 2015. PMID: 26434780 Free PMC article.
Similar articles
-
Cost effectiveness of darunavir/ritonavir in highly treatment-experienced, HIV-1-infected adults in the USA.Pharmacoeconomics. 2010;28 Suppl 1:83-105. doi: 10.2165/11587470-000000000-00000. Pharmacoeconomics. 2010. PMID: 21182346
-
Health benefits, costs, and cost-effectiveness of earlier eligibility for adult antiretroviral therapy and expanded treatment coverage: a combined analysis of 12 mathematical models.Lancet Glob Health. 2014 Jan;2(1):e23-34. doi: 10.1016/S2214-109X(13)70172-4. Epub 2013 Dec 10. Lancet Glob Health. 2014. PMID: 25104632
-
Cost-effectiveness of population-level expansion of highly active antiretroviral treatment for HIV in British Columbia, Canada: a modelling study.Lancet HIV. 2015 Sep;2(9):e393-400. doi: 10.1016/S2352-3018(15)00127-7. Epub 2015 Jul 16. Lancet HIV. 2015. PMID: 26423553 Free PMC article.
-
Implications for a policy of initiating antiretroviral therapy in people diagnosed with human immunodeficiency virus: the CAPRA research programme.Southampton (UK): NIHR Journals Library; 2017 Oct. Southampton (UK): NIHR Journals Library; 2017 Oct. PMID: 29083561 Free Books & Documents. Review.
-
API consensus guidelines for use of antiretroviral therapy in adults (API-ART guidelines). Endorsed by the AIDS Society of India.J Assoc Physicians India. 2006 Jan;54:57-74. J Assoc Physicians India. 2006. PMID: 16649742 Review.
Cited by
-
Do people with different sociodemographic backgrounds value their health differently? Evaluating the role of positional objectivity.Front Public Health. 2023 Dec 13;11:1234320. doi: 10.3389/fpubh.2023.1234320. eCollection 2023. Front Public Health. 2023. PMID: 38162609 Free PMC article.
-
From policy to practice: syndemic and intersectional challenges to ART adherence for transgender women under India's post-test and treat policy.Glob Public Health. 2025 Dec;20(1):2473446. doi: 10.1080/17441692.2025.2473446. Epub 2025 Mar 6. Glob Public Health. 2025. PMID: 40047553
References
-
- Sankalak, status of National AIDS Response (Fourth Edition) New Delhi: NACO; 2022. National AIDS Control Organisation. Ministry of Health & Family Welfare, Government of India.
-
- National AIDS Control Organisation. Ministry of Health & Family Welfare, Government of India. Office memorandum. [accessed on November 8, 2021]. Available from: http://naco.gov.in/sites/default/files/Scan_OM%20CST.pdf .
-
- Rewari BB, Agarwal R, Shastri S, Nagaraja SB, Rathore AS. Adoption of the 2015 World Health Organization guidelines on antiretroviral therapy: Programmatic implications for India. WHO South East Asia J Public Health. 2017;6:90–3. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
