Simplified clinical algorithm for immediate antiretroviral therapy initiation: The HATI [HIV awal (early) Test & Treat in Indonesia] implementation research in Indonesia
- PMID: 37056072
- PMCID: PMC10278919
- DOI: 10.4103/ijmr.ijmr_239_23
Simplified clinical algorithm for immediate antiretroviral therapy initiation: The HATI [HIV awal (early) Test & Treat in Indonesia] implementation research in Indonesia
Abstract
Background & objectives: Although the World Health Organization recommends same day or rapid (< seven days) antiretroviral therapy (ART) initiation, delays in ART initiation remain common due to waiting for laboratory test results. This study employed a simplified clinical algorithm the HATI [HIV Awal (Early) Test & Treat Indonesia]-SAI (Simple ART Initiation) aimed to increase the proportion of ART uptake and decrease the time to ART initiation that can be used in various care settings.
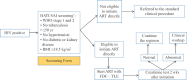
Methods: This study compared the percentage of ART uptake and retention, viral load (VL) suppression and time to ART initiation between the observation and intervention phases among newly diagnosed HIV patients from key populations. As part of the intervention, the newly diagnosed patients underwent screening using a simple form [consisting of data on age, height and weight (for body mass index calculation), questions on the presence of symptoms of HIV stages 1 and 2, tuberculosis, history of diabetes, hypertension and kidney disease], to determine eligibility for immediate ART initiation. Those who met the pre-defined criteria immediately received a combination of tenofovir lamivudine and efavirenz for two weeks. The baseline laboratory examination due to this was moved up to two weeks post ART. Factors significantly associated with ART uptake were also determined and their odds ratios were measured using logistic regression analysis.
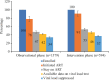
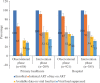
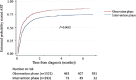
Results: A total of 2173 people newly diagnosed with HIV were recruited, with 1579 and 594 in the observation and intervention phases, respectively. In both phases, the majority were men who have sex with men, who were young (<30 yr old) and employed, with high levels of education. The intervention phase significantly increased the proportion of ART initiation [91%, 95% confidence interval (CI): 89-93% vs. 78%, 95% CI: 76-80%] but did not have any impact on the proportion of six months retention and VL suppression. The intervention also significantly decreased the time to ART initiation from median ± interquartile range: 9±20 days to 2±10 days.
Interpretation & conclusions: The findings of this study suggest that the HATI-SAI intervention increased the uptake and decreased the time for immediate ART initiation. The HATI-SAI provides a simple and safe clinical approach that can readily be adopted in different settings without a costly investment in technology.
Keywords: ART; Early HIV Awal Test and Treat Indonesia; HATI SAI; HIV-AIDS.
Conflict of interest statement
Figures
References
-
- Joint United Nations Programme on HIV/AIDS. Fact sheet –World AIDS Day 2019. Global HIV statistics. 2019. [accessed on November 27, 2022]. Available from: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_... .
-
- Joint United Nations Programme on HIV/AIDS. 90–90–90: Good progress, but the world is off-track for hitting the 2020 targets. [accessed on May 25, 2021]. Available from: https://www.unaids.org/en/resources/presscentre/featurestories/2020/sept... .
-
- Joint United Nations Programme on HIV/AIDS. HIV AIDS Asia Pacific research statistical data information resources –AIDS data hub. 2022. [accessed on June 11, 2020]. Available from: http://aphub.unaids.org/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
