Reperfusion after hypoxia-ischemia exacerbates brain injury with compensatory activation of the anti- ferroptosis system: based on a novel rat model
- PMID: 37056142
- PMCID: PMC10328270
- DOI: 10.4103/1673-5374.369117
Reperfusion after hypoxia-ischemia exacerbates brain injury with compensatory activation of the anti- ferroptosis system: based on a novel rat model
Abstract
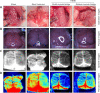
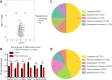
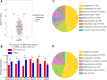
Hypoxic-ischemic encephalopathy, which predisposes to neonatal death and neurological sequelae, has a high morbidity, but there is still a lack of effective prevention and treatment in clinical practice. To better understand the pathophysiological mechanism underlying hypoxic-ischemic encephalopathy, in this study we compared hypoxic-ischemic reperfusion brain injury and simple hypoxic-ischemic brain injury in neonatal rats. First, based on the conventional Rice-Vannucci model of hypoxic-ischemic encephalopathy, we established a rat model of hypoxic-ischemic reperfusion brain injury by creating a common carotid artery muscle bridge. Then we performed tandem mass tag-based proteomic analysis to identify differentially expressed proteins between the hypoxic-ischemic reperfusion brain injury model and the conventional Rice-Vannucci model and found that the majority were mitochondrial proteins. We also performed transmission electron microscopy and found typical characteristics of ferroptosis, including mitochondrial shrinkage, ruptured mitochondrial membranes, and reduced or absent mitochondrial cristae. Further, both rat models showed high levels of glial fibrillary acidic protein and low levels of myelin basic protein, which are biological indicators of hypoxic-ischemic brain injury and indicate similar degrees of damage. Finally, we found that ferroptosis-related Ferritin (Fth1) and glutathione peroxidase 4 were expressed at higher levels in the brain tissue of rats with hypoxic-ischemic reperfusion brain injury than in rats with simple hypoxic-ischemic brain injury. Based on these results, it appears that the rat model of hypoxic-ischemic reperfusion brain injury is more closely related to the pathophysiology of clinical reperfusion. Reperfusion not only aggravates hypoxic-ischemic brain injury but also activates the anti-ferroptosis system.
Keywords: Rice-Vannucci; ferroptosis; hypoxic-ischemic brain injury; hypoxic-ischemic encephalopathy; hypoxic-ischemic reperfusion brain injury; mitochondria; model; proteomic analysis; reperfusion; transmission electron microscopy.
Conflict of interest statement
None
Figures




References
-
- Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, Kapellou O, Levene M, Marlow N, Porter E, Thoresen M, Whitelaw A, Brocklehurst P. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–1358. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

