Exosomes from bone marrow mesenchymal stem cells are a potential treatment for ischemic stroke
- PMID: 37056144
- PMCID: PMC10328279
- DOI: 10.4103/1673-5374.369114
Exosomes from bone marrow mesenchymal stem cells are a potential treatment for ischemic stroke
Abstract
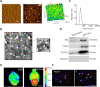
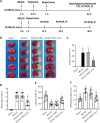
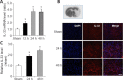
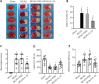
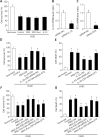
Exosomes derived from human bone marrow mesenchymal stem cells (MSC-Exo) are characterized by easy expansion and storage, low risk of tumor formation, low immunogenicity, and anti-inflammatory effects. The therapeutic effects of MSC-Exo on ischemic stroke have been widely explored. However, the underlying mechanism remains unclear. In this study, we established a mouse model of ischemic brain injury induced by occlusion of the middle cerebral artery using the thread bolt method and injected MSC-Exo into the tail vein. We found that administration of MSC-Exo reduced the volume of cerebral infarction in the ischemic brain injury mouse model, increased the levels of interleukin-33 (IL-33) and suppression of tumorigenicity 2 receptor (ST2) in the penumbra of cerebral infarction, and improved neurological function. In vitro results showed that astrocyte-conditioned medium of cells deprived of both oxygen and glucose, to simulate ischemia conditions, combined with MSC-Exo increased the survival rate of primary cortical neurons. However, after transfection by IL-33 siRNA or ST2 siRNA, the survival rate of primary cortical neurons was markedly decreased. These results indicated that MSC-Exo inhibited neuronal death induced by oxygen and glucose deprivation through the IL-33/ST2 signaling pathway in astrocytes. These findings suggest that MSC-Exo may reduce ischemia-induced brain injury through regulating the IL-33/ST2 signaling pathway. Therefore, MSC-Exo may be a potential therapeutic method for ischemic stroke.
Keywords: IL-33; ST2; astrocytes; bone marrow mesenchymal stem cells; brain injury; exosome; inflammation; ischemic stroke; neurological function; neuron.
Conflict of interest statement
None
Figures

References
-
- Dinarello CA. An IL-1 family member requires caspase-1 processing and signals through the ST2 receptor. Immunity. 2005;23:461–462. - PubMed
LinkOut - more resources
Full Text Sources

