Exosomes derived from human umbilical cord mesenchymal stem cells alleviate Parkinson's disease and neuronal damage through inhibition of microglia
- PMID: 37056150
- PMCID: PMC10328268
- DOI: 10.4103/1673-5374.368300
Exosomes derived from human umbilical cord mesenchymal stem cells alleviate Parkinson's disease and neuronal damage through inhibition of microglia
Abstract
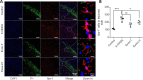
Microglia-mediated inflammatory responses have been shown to play a crucial role in Parkinson's disease. In addition, exosomes derived from mesenchymal stem cells have shown anti-inflammatory effects in the treatment of a variety of diseases. However, whether they can protect neurons in Parkinson's disease by inhibiting microglia-mediated inflammatory responses is not yet known. In this study, exosomes were isolated from human umbilical cord mesenchymal stem cells and injected into a 6-hydroxydopamine-induced rat model of Parkinson's disease. We found that the exosomes injected through the tail vein and lateral ventricle were absorbed by dopaminergic neurons and microglia on the affected side of the brain, where they repaired nigral-striatal dopamine system damage and inhibited microglial activation. Furthermore, in an in vitro cell model, pretreating lipopolysaccharide-stimulated BV2 cells with exosomes reduced interleukin-1β and interleukin-18 secretion, prevented the adoption of pyroptosis-associated morphology by BV2 cells, and increased the survival rate of SH-SY5Y cells. Potential targets for treatment with human umbilical cord mesenchymal stem cells and exosomes were further identified by high-throughput microRNA sequencing and protein spectrum sequencing. Our findings suggest that human umbilical cord mesenchymal stem cells and exosomes are a potential treatment for Parkinson's disease, and that their neuroprotective effects may be mediated by inhibition of excessive microglial proliferation.
Keywords: 6-hydroxydopamine; Parkinson’s disease; dopamine neurons; exosomes; inflammation; mesenchymal stem cells; microglia; pyroptosis.
Conflict of interest statement
None
Figures







References
-
- Ahmed S, Kwatra M, Ranjan Panda S, Murty USN, Naidu VGM. Andrographolide suppresses NLRP3 inflammasome activation in microglia through induction of parkin-mediated mitophagy in in-vitro and in-vivo models of Parkinson disease. Brain Behav Immun. 2021;91:142–158. - PubMed
-
- Avram CM, Brumbach BH, Hiller AL. A report of tamoxifen and Parkinson's disease in a US population and a review of the literature. Mov Disord. 2021;36:1238–1242. - PubMed
LinkOut - more resources
Full Text Sources

