Silicate ions as soluble form of bioactive ceramics alleviate aortic aneurysm and dissection
- PMID: 37056259
- PMCID: PMC10086764
- DOI: 10.1016/j.bioactmat.2022.07.005
Silicate ions as soluble form of bioactive ceramics alleviate aortic aneurysm and dissection
Abstract
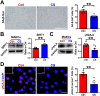
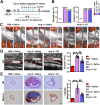
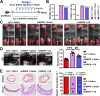
Aortic aneurysm and dissection (AAD) are leading causes of death in the elderly. Recent studies have demonstrated that silicate ions can manipulate multiple cells, especially vascular-related cells. We demonstrated in this study that silicate ions as soluble form of bioactive ceramics effectively alleviated aortic aneurysm and dissection in both Ang II and β-BAPN induced AAD models. Different from the single targeting therapeutic drug approaches, the bioactive ceramic derived approach attributes to the effect of bioactive silicate ions on the inhibition of the AAD progression through regulating the local vascular microenvironment of aorta systematically in a multi-functional way. The in vitro experiments revealed that silicate ions did not only alleviate senescence and inflammation of the mouse aortic endothelial cells, enhance M2 polarization of mouse bone marrow-derived macrophages, and reduce apoptosis of mouse aortic smooth muscle cells, but also regulate their interactions. The in vivo studies further confirm that silicate ions could effectively alleviate senescence, inflammation, and cell apoptosis of aortas, accomplished with reduced aortic dilation, collagen deposition, and elastin laminae degradation. This bioactive ceramic derived therapy provides a potential new treatment strategy in attenuating AAD progression.
Keywords: Aortic aneurysm and dissection; Cell apoptosis; Inflammation; Senescence; Silicate ions.
© 2022 The Authors.
Figures






Similar articles
-
Regulation of immune response by bioactive ions released from silicate bioceramics for bone regeneration.Acta Biomater. 2018 Jan 15;66:81-92. doi: 10.1016/j.actbio.2017.08.044. Epub 2017 Aug 30. Acta Biomater. 2018. PMID: 28864248
-
IL-5 overexpression attenuates aortic dissection by reducing inflammation and smooth muscle cell apoptosis.Life Sci. 2020 Jan 15;241:117144. doi: 10.1016/j.lfs.2019.117144. Epub 2019 Dec 9. Life Sci. 2020. PMID: 31830482
-
A Novel Finding: Macrophages Involved in Inflammation Participate in Acute Aortic Dissection Complicated with Acute Lung Injury.Curr Mol Med. 2017;17(8):568-579. doi: 10.2174/1566524018666180222123518. Curr Mol Med. 2017. PMID: 29473501
-
Targeting regulated cell death in aortic aneurysm and dissection therapy.Pharmacol Res. 2022 Feb;176:106048. doi: 10.1016/j.phrs.2021.106048. Epub 2021 Dec 28. Pharmacol Res. 2022. PMID: 34968685 Review.
-
Programmed cell death in aortic aneurysm and dissection: A potential therapeutic target.J Mol Cell Cardiol. 2022 Feb;163:67-80. doi: 10.1016/j.yjmcc.2021.09.010. Epub 2021 Sep 28. J Mol Cell Cardiol. 2022. PMID: 34597613 Free PMC article. Review.
Cited by
-
3D-printed Sr2ZnSi2O7 scaffold facilitates vascularized bone regeneration through macrophage immunomodulation.Front Bioeng Biotechnol. 2022 Sep 16;10:1007535. doi: 10.3389/fbioe.2022.1007535. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36185424 Free PMC article.
-
3D-printed GelMA/CaSiO3 composite hydrogel scaffold for vascularized adipose tissue restoration.Regen Biomater. 2023 May 8;10:rbad049. doi: 10.1093/rb/rbad049. eCollection 2023. Regen Biomater. 2023. PMID: 37274616 Free PMC article.
-
Therapeutic silicate biomaterials for sarcopenia treatment by inhibiting inflammation and enhancing muscle regeneration through regulation of Sarcolipin/SIRT signaling pathway.Bioact Mater. 2025 Jun 26;51:787-806. doi: 10.1016/j.bioactmat.2025.06.040. eCollection 2025 Sep. Bioact Mater. 2025. PMID: 40809080 Free PMC article.
-
Ion cocktail therapy for myocardial infarction by synergistic regulation of both structural and electrical remodeling.Exploration (Beijing). 2023 Nov 23;4(3):20230067. doi: 10.1002/EXP.20230067. eCollection 2024 Jun. Exploration (Beijing). 2023. PMID: 38939858 Free PMC article.
-
Extracellular vesicles engineering by silicates-activated endothelial progenitor cells for myocardial infarction treatment in male mice.Nat Commun. 2023 Apr 13;14(1):2094. doi: 10.1038/s41467-023-37832-y. Nat Commun. 2023. PMID: 37055411 Free PMC article.
References
-
- United Nations Department Of Economic And Social Affairs, Population Division The 2019 revision of world population Prospects. https://population.un.org/wpp/
LinkOut - more resources
Full Text Sources
Miscellaneous

