Advances in extracellular vesicle functionalization strategies for tissue regeneration
- PMID: 37056271
- PMCID: PMC10087114
- DOI: 10.1016/j.bioactmat.2022.07.022
Advances in extracellular vesicle functionalization strategies for tissue regeneration
Abstract
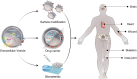
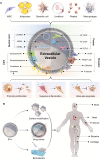
Extracellular vesicles (EVs) are nano-scale vesicles derived by cell secretion with unique advantages such as promoting cell proliferation, anti-inflammation, promoting blood vessels and regulating cell differentiation, which benefit their wide applications in regenerative medicine. However, the in vivo therapeutic effect of EVs still greatly restricted by several obstacles, including the off-targetability, rapid blood clearance, and undesired release. To address these issues, biomedical engineering techniques are vastly explored. This review summarizes different strategies to enhance EV functions from the perspective of drug loading, modification, and combination of biomaterials, and emphatically introduces the latest developments of functionalized EV-loaded biomaterials in different diseases, including cardio-vascular system diseases, osteochondral disorders, wound healing, nerve injuries. Challenges and future directions of EVs are also discussed.
Keywords: Biomaterials; Drug delivery; Extracellular vesicle; Modification; Regenerative medicine.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures









References
Publication types
LinkOut - more resources
Full Text Sources

