Inhibition of Gluconeogenesis by Boldine in the Perfused Liver: Therapeutical Implication for Glycemic Control
- PMID: 37056327
- PMCID: PMC10089784
- DOI: 10.1155/2023/1283716
Inhibition of Gluconeogenesis by Boldine in the Perfused Liver: Therapeutical Implication for Glycemic Control
Abstract
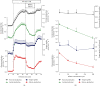
The alkaloid boldine occurs in the Chilean boldo tree (Peumus boldus). It acts as a free radical scavenger and controls glycemia in diabetic rats. Various mechanisms have been proposed for this effect, including inhibited glucose absorption, stimulated insulin secretion, and increased expression of genes involved in glycemic control. Direct effects on glucose synthesis and degradation were not yet measured. To fill this gap, the present study is aimed at ensuring several metabolic pathways linked to glucose metabolism (e.g., gluconeogenesis) in the isolated perfused rat liver. In order to address mechanistic issues, energy transduction in isolated mitochondria and activities of gluconeogenic key enzymes in tissue preparations were also measured. Boldine diminished mitochondrial ROS generation, with no effect on energy transduction in isolated mitochondria. It inhibited, however, at least three enzymes of the gluconeogenic pathway, namely, phosphoenolpyruvate carboxykinase, fructose-bisphosphatase-1, and glucose 6-phosphatase, starting at concentrations below 50 μM. Consistently, in the perfused liver, boldine decreased lactate-, alanine-, and fructose-driven gluconeogenesis with IC50 values of 71.9, 85.2, and 83.6 μM, respectively. Conversely, the compound also increased glycolysis from glycogen-derived glucosyl units. The hepatic ATP content was not affected by boldine. It is proposed that the direct inhibition of hepatic gluconeogenesis by boldine, combined with the increase of glycolysis, could be an important event behind the diminished hyperglycemia observed in boldine-treated diabetic rats.
Copyright © 2023 Laís Cristina Lima Silva et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures






Similar articles
-
Influence of tamoxifen on gluconeogenesis and glycolysis in the perfused rat liver.Chem Biol Interact. 2011 Aug 15;193(1):22-33. doi: 10.1016/j.cbi.2011.04.010. Epub 2011 May 4. Chem Biol Interact. 2011. PMID: 21570382
-
Protective effect of boldine on oxidative mitochondrial damage in streptozotocin-induced diabetic rats.Pharmacol Res. 2000 Oct;42(4):361-71. doi: 10.1006/phrs.2000.0705. Pharmacol Res. 2000. PMID: 10987997
-
Induction and suppression of the key enzymes of glycolysis and gluconeogenesis in isolated perfused rat liver in response to glucose, fructose and lactate.Biochem J. 1973 May;134(1):143-56. doi: 10.1042/bj1340143. Biochem J. 1973. PMID: 4353083 Free PMC article.
-
The role of cyclic AMP in rapid and long-term regulation of gluconeogenesis and glycolysis.Adv Second Messenger Phosphoprotein Res. 1988;22:175-91. Adv Second Messenger Phosphoprotein Res. 1988. PMID: 2852023 Review.
-
An Overview of Chemistry, Kinetics, Toxicity and Therapeutic Potential of Boldine in Neurological Disorders.Neurochem Res. 2023 Nov;48(11):3283-3295. doi: 10.1007/s11064-023-03992-y. Epub 2023 Jul 18. Neurochem Res. 2023. PMID: 37462836 Review.
Cited by
-
Pharmacology of boldine: summary of the field and update on recent advances.Front Pharmacol. 2024 Sep 13;15:1427147. doi: 10.3389/fphar.2024.1427147. eCollection 2024. Front Pharmacol. 2024. PMID: 39346563 Free PMC article. Review.
References
-
- Nabavi S. M., Uriarte E., Fontenla J. A., Rastrelli L., Sobarzo-Sanchez E. Aporphine alkaloids and their antioxidant medical application: from antineoplastic agents to motor dysfunction diseases. Current Organic Chemistry . 2017;21(4):342–347. doi: 10.2174/1385272820666161017165735. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

