A flexible and versatile system for multi-color fiber photometry and optogenetic manipulation
- PMID: 37056369
- PMCID: PMC10088095
- DOI: 10.1016/j.crmeth.2023.100418
A flexible and versatile system for multi-color fiber photometry and optogenetic manipulation
Abstract
Here, we present simultaneous fiber photometry recordings and optogenetic stimulation based on a multimode fused fiber coupler for both light delivery and collection without the need for dichroic beam splitters. In combination with a multi-color light source and appropriate optical filters, our approach offers remarkable flexibility in experimental design and facilitates the exploration of new molecular tools in vivo at minimal cost. We demonstrate straightforward re-configuration of the setup to operate with green, red, and near-infrared calcium indicators with or without simultaneous optogenetic stimulation and further explore the multi-color photometry capabilities of the system. The ease of assembly, operation, characterization, and customization of this platform holds the potential to foster the development of experimental strategies for multi-color fused fiber photometry combined with optogenetics far beyond its current state.
Keywords: all-optical; fiber photometry; fused fiber coupler; genetically encoded indicators; hippocampus; in vivo; mouse brain; neuroscience; open source; optogenetics.
© 2023 The Author(s).
Conflict of interest statement
The authors have a patent application pending for the use of fused fiber optics for bidirectional communication with electrically excitable cells (50%/25%/25% by A.F./A.D./J.S.W.).
Figures
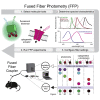




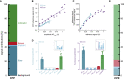

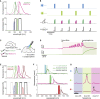
Comment in
-
Fused fiber couplers for fiber photometry.Cell Rep Methods. 2023 Mar 27;3(3):100439. doi: 10.1016/j.crmeth.2023.100439. eCollection 2023 Mar 27. Cell Rep Methods. 2023. PMID: 37056372 Free PMC article.
Similar articles
-
Multi-Frequency Interpolation X-talk Removal Algorithm: Enabling Combinations of Concurrent Optogenetics and Lock-in Amplification Fiber Photometry via Removal of Optogenetic Stimulation Crosstalk.ACS Chem Neurosci. 2025 May 7;16(9):1694-1709. doi: 10.1021/acschemneuro.4c00632. Epub 2025 Apr 14. ACS Chem Neurosci. 2025. PMID: 40228799
-
Fused fiber couplers for fiber photometry.Cell Rep Methods. 2023 Mar 27;3(3):100439. doi: 10.1016/j.crmeth.2023.100439. eCollection 2023 Mar 27. Cell Rep Methods. 2023. PMID: 37056372 Free PMC article.
-
High-density multi-fiber photometry for studying large-scale brain circuit dynamics.Nat Methods. 2019 Jun;16(6):553-560. doi: 10.1038/s41592-019-0400-4. Epub 2019 May 13. Nat Methods. 2019. PMID: 31086339
-
Multimodal Functional Neuroimaging by Simultaneous BOLD fMRI and Fiber-Optic Calcium Recordings and Optogenetic Control.Mol Imaging Biol. 2018 Apr;20(2):171-182. doi: 10.1007/s11307-017-1130-6. Mol Imaging Biol. 2018. PMID: 29027094 Review.
-
Calcium Imaging in Vivo: How to Correctly Select and Apply Fiber Optic Photometric Indicators.Organogenesis. 2025 Dec;21(1):2489667. doi: 10.1080/15476278.2025.2489667. Epub 2025 Apr 5. Organogenesis. 2025. PMID: 40186873 Free PMC article. Review.
Cited by
-
Obesity-induced inflammation: connecting the periphery to the brain.Nat Metab. 2024 Jul;6(7):1237-1252. doi: 10.1038/s42255-024-01079-8. Epub 2024 Jul 12. Nat Metab. 2024. PMID: 38997442 Review.
-
Sensitive multicolor indicators for monitoring norepinephrine in vivo.Nat Methods. 2023 Sep;20(9):1426-1436. doi: 10.1038/s41592-023-01959-z. Epub 2023 Jul 20. Nat Methods. 2023. PMID: 37474807 Free PMC article.
-
Multi-Frequency Interpolation X-talk Removal Algorithm: Enabling Combinations of Concurrent Optogenetics and Lock-in Amplification Fiber Photometry via Removal of Optogenetic Stimulation Crosstalk.ACS Chem Neurosci. 2025 May 7;16(9):1694-1709. doi: 10.1021/acschemneuro.4c00632. Epub 2025 Apr 14. ACS Chem Neurosci. 2025. PMID: 40228799
-
Identifying behavioral links to neural dynamics of multifiber photometry recordings in a mouse social behavior network.bioRxiv [Preprint]. 2024 Jan 6:2023.12.25.573308. doi: 10.1101/2023.12.25.573308. bioRxiv. 2024. Update in: J Neural Eng. 2024 Jun 25;21(3). doi: 10.1088/1741-2552/ad5702. PMID: 38234793 Free PMC article. Updated. Preprint.
-
A comparison of viral strategies and model systems to target norepinephrine neurons in the locus coeruleus reveals high variability in transgene expression patterns.PLoS Biol. 2025 Jul 7;23(7):e3003228. doi: 10.1371/journal.pbio.3003228. eCollection 2025 Jul. PLoS Biol. 2025. PMID: 40623068 Free PMC article.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

