Association of biological sex with clinical outcomes and biomarkers of Alzheimer's disease in adults with Down syndrome
- PMID: 37056479
- PMCID: PMC10088472
- DOI: 10.1093/braincomms/fcad074
Association of biological sex with clinical outcomes and biomarkers of Alzheimer's disease in adults with Down syndrome
Abstract
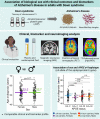
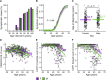
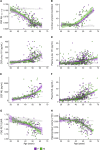
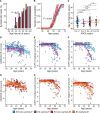
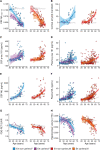
The study of sex differences in Alzheimer's disease is increasingly recognized as a key priority in research and clinical development. People with Down syndrome represent the largest population with a genetic link to Alzheimer's disease (>90% in the 7th decade). Yet, sex differences in Alzheimer's disease manifestations have not been fully investigated in these individuals, who are key candidates for preventive clinical trials. In this double-centre, cross-sectional study of 628 adults with Down syndrome [46% female, 44.4 (34.6; 50.7) years], we compared Alzheimer's disease prevalence, as well as cognitive outcomes and AT(N) biomarkers across age and sex. Participants were recruited from a population-based health plan in Barcelona, Spain, and from a convenience sample recruited via services for people with intellectual disabilities in England and Scotland. They underwent assessment with the Cambridge Cognitive Examination for Older Adults with Down Syndrome, modified cued recall test and determinations of brain amyloidosis (CSF amyloid-β 42 / 40 and amyloid-PET), tau pathology (CSF and plasma phosphorylated-tau181) and neurodegeneration biomarkers (CSF and plasma neurofilament light, total-tau, fluorodeoxyglucose-PET and MRI). We used within-group locally estimated scatterplot smoothing models to compare the trajectory of biomarker changes with age in females versus males, as well as by apolipoprotein ɛ4 carriership. Our work revealed similar prevalence, age at diagnosis and Cambridge Cognitive Examination for Older Adults with Down Syndrome scores by sex, but males showed lower modified cued recall test scores from age 45 compared with females. AT(N) biomarkers were comparable in males and females. When considering apolipoprotein ɛ4, female ɛ4 carriers showed a 3-year earlier age at diagnosis compared with female non-carriers (50.5 versus 53.2 years, P = 0.01). This difference was not seen in males (52.2 versus 52.5 years, P = 0.76). Our exploratory analyses considering sex, apolipoprotein ɛ4 and biomarkers showed that female ɛ4 carriers tended to exhibit lower CSF amyloid-β 42/amyloid-β 40 ratios and lower hippocampal volume compared with females without this allele, in line with the clinical difference. This work showed that biological sex did not influence clinical and biomarker profiles of Alzheimer's disease in adults with Down syndrome. Consideration of apolipoprotein ɛ4 haplotype, particularly in females, may be important for clinical research and clinical trials that consider this population. Accounting for, reporting and publishing sex-stratified data, even when no sex differences are found, is central to helping advance precision medicine.
Keywords: Alzheimer’s disease; Down syndrome; gender; precision medicine; sex.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
O.B. reported receiving personal fees from ADx NeuroSciences outside the submitted work. H.Z. declares that he has served on scientific advisory boards and/or as a consultant for Abbvie, Alector, Annexon, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Pinteon Therapeutics, Red Abbey Labs, Passage Bio, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen and Roche and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, which is a part of the GU Ventures Incubator Program, outside the submitted work. K.B. declares that he has served as a consultant, on advisory boards, or data monitoring committees for Abcam, Axon, BioArctic, Biogen, JOMDD/Shimadzu, Julius Clinical, Lilly, MagQu, Novartis, Pharmatrophix, Prothena, Roche Diagnostics and Siemens Healthineers and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, which is a part of the GU Ventures Incubator Program, outside the submitted work. D.A. reported receiving personal fees for advisory board services and/or speaker honoraria from Fujirebio-Europe, Roche, Nutricia, Krka Farmacéutica and Esteve, outside the submitted work. A.L. has served as a consultant or on advisory boards for Fujirebio-Europe, Roche, Biogen and Nutricia, outside the submitted work. J.F. reported receiving personal fees for service on the advisory boards, adjudication committees or speaker honoraria from AC Immune, Novartis, Lundbeck, Roche, Fujirebio and Biogen, outside the submitted work. O.B., D.A., A.L. and J.F. report holding a patent for markers of synaptopathy in neurodegenerative disease (licensed to ADx, EPI8382175.0). No other competing interests were reported. References1HentzenNB, FerrettiMT, SantuccioneA, et alMapping of European activities on the integration of sex and gender factors in neurology and neuroscience. Eur J Neurol. 2022;29(9):2572–2579.2Spires-JonesTL. Let's talk about sex (in translational neuroscience). Brain Commun. 2022;4(2):fcac028.3GauthierS, Rosa-NetoP, MoraisJA, WebsterC. World Alzheimer Report 2021: Journey through the diagnosis of dementia. 2021.4FerrettiMT, IulitaMF, CavedoE, et alSex differences in Alzheimer disease—The gateway to precision medicine. Nat Rev Neurol. 2018;14(8):457–469.299854745NebelRA, AggarwalNT, BarnesLL, et alUnderstanding the impact of sex and gender in Alzheimer's disease: A call to action. Alzheimers Dement. 2018;14(9):1171–1183.299074236McCarreyAC, AnY, Kitner-TrioloMH, FerrucciL, ResnickSM. Sex differences in cognitive trajectories in clinically normal older adults. Psychol Aging. 2016;31(2):166–175.267967927SundermannEE, MakiP, BiegonA, et alSex-specific norms for verbal memory tests may improve diagnostic accuracy of amnestic MCI. Neurology. 2019;93(20):e1881–e1889.315977088DigmaLA, MadsenJR, RissmanRA, et alWomen can bear a bigger burden: Ante- and post-mortem evidence for reserve in the face of tau. Brain Commun. 2020;2(1):fcaa025.9LawsKR, IrvineK, GaleTM. Sex differences in cognitive impairment in Alzheimer's disease. World J Psychiatry. 2016;6(1):54–65.2701459810SohnD, ShpanskayaK, LucasJE, et alSex differences in cognitive decline in subjects with high likelihood of mild cognitive impairment due to Alzheimer's disease. Sci Rep. 2018;8(1):7490.2974859811LinKA, ChoudhuryKR, RathakrishnanBG, et alMarked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement (N Y). 2015;1(2):103–110.2645138612MielkeMM. Consideration of sex differences in the measurement and interpretation of Alzheimer disease-related biofluid-based biomarkers. J Appl Lab Med. 2020;5(1):158–169.3181107313BuckleyRF, MorminoEC, RabinJS, et alSex differences in the association of global amyloid and regional tau deposition measured by positron emission tomography in clinically normal older adults. JAMA Neurol. 2019;76(5):542–551.3071507814BuckleyRF, ScottMR, JacobsHIL, et alSex mediates relationships between regional tau pathology and cognitive decline. Ann Neurol. 2020;88(5):921–932.3279936715EdwardsL, La JoieR, IaccarinoL, et alMultimodal neuroimaging of sex differences in cognitively impaired patients on the Alzheimer's continuum: Greater tau-PET retention in females. Neurobiol Aging. 2021;105:86–98.3404906216LiesingerAM, Graff-RadfordNR, DuaraR, et alSex and age interact to determine clinicopathologic differences in Alzheimer's disease. Acta Neuropathol. 2018;136(6):873–885.3021993917OveisgharanS, ArvanitakisZ, YuL, FarfelJ, SchneiderJA, BennettDA. Sex differences in Alzheimer's disease and common neuropathologies of aging. Acta Neuropathol. 2018;136(6):887–900.3033407418BridelC, van WieringenWN, ZetterbergH, et alDiagnostic value of cerebrospinal fluid neurofilament light protein in neurology: A systematic review and meta-analysis. JAMA Neurol. 2019;76(9):1035–1048.3120616019NeuSC, PaJ, KukullW, et alApolipoprotein E genotype and sex risk factors for Alzheimer disease: A meta-analysis. JAMA Neurol. 2017;74(10):1178–1189.2884675720SampedroF, VilaplanaE, de LeonMJ, et alAPOE-by-sex interactions on brain structure and metabolism in healthy elderly controls. Oncotarget. 2015;6(29):26663–26674.2639722621HohmanTJ, DumitrescuL, BarnesLL, et alSex-specific association of apolipoprotein E with cerebrospinal fluid levels of tau. JAMA Neurol. 2018;75(8):989–998.2980102422WangYT, PascoalTA, TherriaultJ, et alInteractive rather than independent effect of APOE and sex potentiates tau deposition in women. Brain Commun. 2021;3(2):fcab126.23YanS, ZhengC, ParanjpeMD, et alSex modifies APOE epsilon4 dose effect on brain tau deposition in cognitively impaired individuals. Brain. 2021;144(10):3201–3211.3387681524McCarronM, McCallionP, ReillyE, DunneP, CarrollR, MulryanN. A prospective 20-year longitudinal follow-up of dementia in persons with Down syndrome. J Intellect Disabil Res. 2017;61(9):843–852.2866456125ForteaJ, VilaplanaE, Carmona-IraguiM, et alClinical and biomarker changes of Alzheimer's disease in adults with Down syndrome: A cross-sectional study. Lancet. 2020;395(10242):1988–1997.3259333626SnyderHM, BainLJ, BrickmanAM, et alFurther understanding the connection between Alzheimer's disease and Down syndrome. Alzheimers Dement. 2020;16(7):1065–1077.3254431027LemereCA, BlusztajnJK, YamaguchiH, WisniewskiT, SaidoTC, SelkoeDJ. Sequence of deposition of heterogeneous amyloid beta-peptides and APO E in Down syndrome: Implications for initial events in amyloid plaque formation. Neurobiol Dis. 1996;3(1):16–32.917391028WisniewskiKE, WisniewskiHM, WenGY. Occurrence of neuropathological changes and dementia of Alzheimer's disease in Down's syndrome. Ann Neurol. 1985;17(3):278–282.315826629ForteaJ, ZamanSH, HartleyS, RafiiMS, HeadE, Carmona-IraguiM. Alzheimer's disease associated with Down syndrome: A genetic form of dementia. Lancet Neurol. 2021;20(11):930–942.3468763730BatemanRJ, XiongC, BenzingerTL, et alClinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367(9):795–804.2278403631RafiiMS, AncesBM, SchupfN, et alThe AT(N) framework for Alzheimer's disease in adults with Down syndrome. Alzheimers Dement (Amst). 2020;12(1):e12062.32SchupfN, KapellD, NightingaleB, RodriguezA, TyckoB, MayeuxR. Earlier onset of Alzheimer's disease in men with Down syndrome. Neurology. 1998;50(4):991–995.956638433LaiF, KammannE, RebeckGW, AndersonA, ChenY, NixonRA. APOE genotype and gender effects on Alzheimer disease in 100 adults with Down syndrome. Neurology. 1999;53(2):331–336.1043042234SinaiA, MokryszC, BernalJ, et alPredictors of age of diagnosis and survival of Alzheimer's disease in Down syndrome. J Alzheimers Dis. 2018;61(2):717–728.2922686835LaiF, MhatrePG, YangY, WangMC, SchupfN, RosasHD. Sex differences in risk of Alzheimer's disease in adults with Down syndrome. Alzheimers Dement (Amst). 2020;12(1):e12084.36MhatrePG, LeeJH, PangD, et alThe association between sex and risk of Alzheimer's disease in adults with Down syndrome. J Clin Med. 2021;10(13):2966.3427945037RafiiMS, ZamanS, HandenBL. Integrating biomarker outcomes into clinical trials for Alzheimer's disease in Down syndrome. J Prev Alzheimers Dis. 2021;8(1):48–51.3333622438AnnusT, WilsonLR, HongYT, et alThe pattern of amyloid accumulation in the brains of adults with Down syndrome. Alzheimers Dementia. 2016;12(5):538–545.39AlcoleaD, ClarimonJ, Carmona-IraguiM, et alThe Sant Pau initiative on neurodegeneration (SPIN) cohort: A data set for biomarker discovery and validation in neurodegenerative disorders. Alzheimers Dement (N Y). 2019;5:597–609.3165001640AylwardEH, BurtDB, ThorpeLU, LaiF, DaltonA. Diagnosis of dementia in individuals with intellectual disability. J Intellect Disabil Res. 1997;41(Pt 2):152–164.916192741Esteba-CastilloS, Dalmau-BuenoA, Ribas-VidalN, Vila-AlsinaM, Novell-AlsinaR, Garcia-AlbaJ. [Adaptation and validation of CAMDEX-DS (Cambridge examination for mental disorders of older people with Down's syndrome and others with intellectual disabilities) in Spanish population with intellectual disabilities]. Rev Neurol. 2013;57(8):337–346. Adaptacion y validacion del Cambridge Examination for Mental Disorders of Older People with Down's Syndrome and Others with Intellectual Disabilities (CAMDEX-DS) en poblacion espanola con discapacidad intelectual.2408188842BenejamB, VidelaL, VilaplanaE, et alDiagnosis of prodromal and Alzheimer's disease dementia in adults with Down syndrome using neuropsychological tests. Alzheimers Dement (Amst). 2020;12(1):e12047.43ForteaJ, Carmona-IraguiM, BenejamB, et alPlasma and CSF biomarkers for the diagnosis of Alzheimer's disease in adults with Down syndrome: A cross-sectional study. Lancet Neurol. 2018;17(10):860–869.3017262444AlcoleaD, PeguerolesJ, MunozL, et alAgreement of amyloid PET and CSF biomarkers for Alzheimer's disease on Lumipulse. Ann Clin Transl Neurol. 2019;6(9):1815–1824.3146408845DelabyC, EstellesT, ZhuN, et alThe Abeta1–42/Abeta1–40 ratio in CSF is more strongly associated to tau markers and clinical progression than Abeta1–42 alone. Alzheimers Res Ther. 2022;14(1):20.3510535146LleoA, ZetterbergH, PeguerolesJ, et alPhosphorylated tau181 in plasma as a potential biomarker for Alzheimer's disease in adults with Down syndrome. Nat Commun. 2021;12(1):4304.3426203047LandauSM, HarveyD, MadisonCM, et alAssociations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging. 2011;32(7):1207–1218.1966083448AvantsBB, TustisonNJ, SongG, CookPA, KleinA, GeeJC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3):2033–2044.2085119149ZammitMD, LaymonCM, BetthauserTJ, et alAmyloid accumulation in Down syndrome measured with amyloid load. Alzheimers Dement (Amst). 2020;12(1):e12020.50KlunkWE, KoeppeRA, PriceJC, et alThe Centiloid project: Standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement. 2015;11(1):1–15e1–4.2544385751HastieTJ. Generalized additive models. In: Chambers JM, Hastie TJ, eds. Statistical models in S. Wadsworth & Brooks; 1992:309–376.52FerrettiMT, MartinkovaJ, BiskupE, et alSex and gender differences in Alzheimer's disease: Current challenges and implications for clinical practice: Position paper of the Dementia and Cognitive Disorders Panel of the European Academy of Neurology. Eur J Neurol. 2020;27(6):928–943.3205634753RaghavanR, Khin-NuC, BrownAG, et alGender differences in the phenotypic expression of Alzheimer's disease in Down's syndrome (trisomy 21). Neuroreport. 1994;5(11):1393–1396.791920754SchupfN, PangD, PatelBN, et alOnset of dementia is associated with age at menopause in women with Down's syndrome. Ann Neurol. 2003;54(4):433–438.1452065355JansenWJ, OssenkoppeleR, KnolDL, et alPrevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA. 2015;313(19):1924–1938.2598846256BuckleyRF, MorminoEC, AmariglioRE, et alSex, amyloid, and APOE epsilon4 and risk of cognitive decline in preclinical Alzheimer's disease: Findings from three well-characterized cohorts. Alzheimers Dement. 2018;14(9):1193–1203.2980354157GurRC, TuretskyBI, MatsuiM, et alSex differences in brain gray and white matter in healthy young adults: Correlations with cognitive performance. J Neurosci. 1999;19(10):4065–4072.1023403458WisniewskiKE, DaltonAJ, McLachlanC, WenGY, WisniewskiHM. Alzheimer's disease in Down's syndrome: Clinicopathologic studies. Neurology. 1985;35(7):957–961.315997459MannDM. The pathological association between Down syndrome and Alzheimer disease. Mech Ageing Dev. 1988;43(2):99–136.296944160BallMJ, NuttallK. Topography of neurofibrillary tangles and granulovacuoles in hippocampi of patients with Down's syndrome: Quantitative comparison with normal ageing and Alzheimer's disease. Neuropathol Appl Neurobiol. 1981;7(1):13–20.645330161MotteJ, WilliamsRS. Age-related changes in the density and morphology of plaques and neurofibrillary tangles in Down syndrome brain. Acta Neuropathol. 1989;77(5):535–546.252415062Vila-CastelarC, Guzman-VelezE, Pardilla-DelgadoE, et alExamining sex differences in markers of cognition and neurodegeneration in autosomal dominant Alzheimer's disease: Preliminary findings from the Colombian Alzheimer's prevention initiative biomarker study. J Alzheimers Dis. 2020;77(4):1743–1753.3292506763OssenkoppeleR, LyooCH, Jester-BromsJ, et alAssessment of demographic, genetic, and imaging variables associated with brain resilience and cognitive resilience to pathological tau in patients with Alzheimer disease. JAMA Neurol. 2020;77(5):632–642.3209154964BejaninA, IulitaMF, VilaplanaE, et alAssociation of apolipoprotein E varepsilon4 allele with clinical and multimodal biomarker changes of Alzheimer disease in adults with Down syndrome. JAMA Neurol. 2021;78(8):937–947.3422804265CacciagliaR, SalvadoG, MolinuevoJL, et alAge, sex and APOE-epsilon4 modify the balance between soluble and fibrillar beta-amyloid in non-demented individuals: Topographical patterns across two independent cohorts. Mol Psychiatry. 2022;27(4):2010–2018.3523695866ChoSH, WooS, KimC, et alDisease progression modelling from preclinical Alzheimer's disease (AD) to AD dementia. Sci Rep. 2021;11(1):4168.3360301567MathysH, Davila-VelderrainJ, PengZ, et alSingle-cell transcriptomic analysis of Alzheimer's disease. Nature. 2019;570(7761):332–337.3104269768AndrewsEJ, MartiniAC, HeadE. Exploring the role of sex differences in Alzheimer's disease pathogenesis in Down syndrome. Front Neurosci. 2022;16:954999.69SchupfN, ZigmanW, KapellD, LeeJH, KlineJ, LevinB. Early menopause in women with Down's syndrome. J Intellect Disabil Res. 1997;41(Pt 3):264–267.921907670CoppusA, EvenhuisH, VerberneGJ, et alEarly age at menopause is associated with increased risk of dementia and mortality in Down syndrome. J Appl Res Intellect Disabil. 2010;23(5):408–408.71AlmeyA, MilnerTA, BrakeWG. Estrogen receptors in the central nervous system and their implication for dopamine-dependent cognition in females. Horm Behav. 2015;74:125–138.2612229472SpampinatoSF, MolinaroG, MerloS, et alEstrogen receptors and type 1 metabotropic glutamate receptors are interdependent in protecting cortical neurons against beta-amyloid toxicity. Mol Pharmacol. 2012;81(1):12–20.2198425373LiangK, YangL, YinC, et alEstrogen stimulates degradation of beta-amyloid peptide by up-regulating neprilysin. J Biol Chem. 2010;285(2):935–942.1989748574AmtulZ, WangL, WestawayD, RozmahelRF. Neuroprotective mechanism conferred by 17beta-estradiol on the biochemical basis of Alzheimer's disease. Neuroscience. 2010;169(2):781–786.2049392875Batallan BurrowesAA, OlajideOJ, IasenzaIA, ShamsWM, CarterF, ChapmanCA. Ovariectomy reduces cholinergic modulation of excitatory synaptic transmission in the rat entorhinal cortex. PLoS One. 2022;17(8):e0271131.76TaxierLR, PhilippiSM, FleischerAW, YorkJM, LaDuMJ, FrickKM. APOE4 homozygote females are resistant to the beneficial effects of 17beta-estradiol on memory and CA1 dendritic spine density in the EFAD mouse model of Alzheimer's disease. Neurobiol Aging. 2022;118:13–24.3584310977BrintonRD. Cellular and molecular mechanisms of estrogen regulation of memory function and neuroprotection against Alzheimer's disease: Recent insights and remaining challenges. Learn Mem. 2001;8(3):121–133.1139063278Garcia-SeguraLM, AzcoitiaI, DonCarlosLL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63(1):29–60.1104041779CosgraveMP, TyrrellJ, McCarronM, GillM, LawlorBA. Age at onset of dementia and age of menopause in women with Down's syndrome. J Intellect Disabil Res. 1999;43(Pt 6):461–465.1062236180SchupfN, WinstenS, PatelB, et alBioavailable estradiol and age at onset of Alzheimer's disease in postmenopausal women with Down syndrome. Neurosci Lett. 2006;406(3):298–302.1692606781SchupfN, LeeJH, WeiM, et alEstrogen receptor-alpha variants increase risk of Alzheimer's disease in women with Down syndrome. Dement Geriatr Cogn Disord. 2008;25(5):476–482.1840836682de Gonzalo-CalvoD, BarroetaI, NanMN, et alEvaluation of biochemical and hematological parameters in adults with Down syndrome. Sci Rep. 2020;10(1):13755.3279261983StartinCM, D'SouzaH, BallG, et alHealth comorbidities and cognitive abilities across the lifespan in Down syndrome. J Neurodev Disord. 2020;12(1):4.3197369784IulitaMF, GarzonD, ChristensenMK, et alAssociation of Alzheimer disease with life expectancy in people with Down syndrome. JAMA Network Open. 2022;5(5):e2212910.85PatelBN, SeltzerGB, WuHS, SchupfN. Effect of menopause on cognitive performance in women with Down syndrome. Neuroreport. 2001;12(12):2659–2662.1152294386CoppusAMW, EvenhuisHM, VerberneGJ, et alEarly age at menopause is associated with increased risk of dementia and mortality in women with Down syndrome. J Alzheimers Dis. 2010;19(2):545–550.20110600
Figures
References
-
- Hentzen NB, Ferretti MT, Santuccione A, et al. . Mapping of European activities on the integration of sex and gender factors in neurology and neuroscience. Eur J Neurol. 2022;29(9):2572–2579. - PubMed
-
- Gauthier S, Rosa-Neto P, Morais JA, Webster C. World Alzheimer Report 2021: Journey through the diagnosis of dementia. 2021.
-
- Ferretti MT, Iulita MF, Cavedo E, et al. . Sex differences in Alzheimer disease—The gateway to precision medicine. Nat Rev Neurol. 2018;14(8):457–469. - PubMed
