Cytokine engineered NK-92 therapy to improve persistence and anti-tumor activity
- PMID: 37056568
- PMCID: PMC10086201
- DOI: 10.7150/thno.79942
Cytokine engineered NK-92 therapy to improve persistence and anti-tumor activity
Abstract
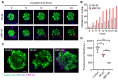
Natural killer (NK) cells are an attractive cell source in cancer immunotherapy due to their potent antitumor ability and promising safety for allogenic applications. However, the clinical outcome of NK cell therapy has been limited due to poor persistence and loss of activity in the cytokine-deficient tumor microenvironment. Benefits from exogenous administration of soluble interleukin-2 (IL-2) to stimulate the activity of NK cells have not been significant due to cytokine consumption and activation of other immune cells, compromising both efficacy and safety. Methods: To overcome these drawbacks, we developed a novel membrane-bound protein (MBP) technology to express IL-2 on the surface of NK-92 cells (MBP NK) inducing autocrine signal for proliferation without IL-2 supplementation. Results: The MBP NK cells exhibited not only improved proliferation in IL-2 deficient conditions but also stronger secretion of cytolytic granules leading to enhanced anti-tumor activity both in vitro and in vivo. Furthermore, the experiment with a spheroid solid tumor model exhibited enhanced infiltration by MBP NK cells creating higher local effector-to-target ratio for efficient tumor killing. These results suggest MBP technology can be an effective utility for NK-92 cell engineering to increase anti-tumor activity and reduce potential adverse effects, providing a higher therapeutic index in clinical applications.
Keywords: interleukin-2; membrane-bound protein (MBP); microwell array chip; natural killer cell; self-activation; tumor-infiltrating lymphocytes.
© The author(s).
Conflict of interest statement
Competing Interests: H.Y.S., J.L., S.J and M.S.K. have financial interests in CTCELLS, Inc. All other authors declare that they have no competing interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

