High versus Medium Dose of Inhaled Corticosteroid in Chronic Obstructive Lung Disease: A Systematic Review and Meta-Analysis
- PMID: 37056683
- PMCID: PMC10086393
- DOI: 10.2147/COPD.S401736
High versus Medium Dose of Inhaled Corticosteroid in Chronic Obstructive Lung Disease: A Systematic Review and Meta-Analysis
Abstract
Background: Inhaled corticosteroids (ICSs) combined with bronchodilators have been identified to improve outcomes in COPD but also to be associated with certain adverse effects.
Objective: We performed a systematic review and meta-analysis to compile and summarize data on the efficacy and safety of dosing levels (high versus medium/low) of ICS alongside ancillary bronchodilators following PRISMA guidelines.
Data sources: Medline and Embase were systematically searched until December 2021. Randomized, clinical trials (RCTs) that met predefined inclusion criteria were included.
Data extraction: Risk ratios (RRs) with 95% confidence intervals (CI) were extracted. Any acute exacerbation of COPD (AECOPD) risk was chosen as the primary efficacy outcome, mortality rate as the primary safety outcome, moderate/severe AECOPD risk as the secondary efficacy outcome and pneumonia risk as the secondary safety outcome. Subgroup analyses of individual ICS agents, of patients with baseline moderate/severe/very severe COPD and of patients with recent COPD exacerbation history were also performed. A random-effects model was used.
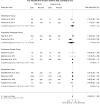
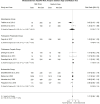
Results: We included 13 RCTs in our study. No data on low doses were included in the analysis. High dose ICS was not associated with a statistically significant difference in any AECOPD risk (RR: 0.98, 95% CI: 0.91-1.05, I2: 41.3%), mortality rate (RR: 0.99, 95% CI: 0.75-1.32, I2: 0.0%), moderate/severe AECOPD risk (RR: 1.01, 95% CI: 0.96-1.06, I2: 0.0%) or pneumonia risk (RR: 1.07, 95% CI: 0.86 -1.33, I2: 9.3%) compared to medium dose ICS. The same trend was identified with the several subgroup analyses.
Conclusion: Our study collected RCTs investigating the optimal dosing level of ICS prescribed alongside ancillary bronchodilators to patients with COPD. We identified that the high ICS dose neither reduces AECOPD risk and mortality rates nor increases pneumonia risk relative to the medium dose.
Keywords: acute COPD exacerbation; chronic obstructive lung disease; inhaled corticosteroids; mortality; pneumonia.
© 2023 Archontakis Barakakis et al.
Conflict of interest statement
FJM reports grants from NHLBI, National Institutes of Health, personal fees from Continuing Education, personal fees from Forest Laboratories, Janssen, GlaxoSmithKline, Nycomed/Takeda, AstraZeneca, Boehringer Ingelheim, Bellerophon (formerly Ikaria), Genentech, Novartis, Pearl, Roche, Sunovion, Theravance, CME Incite, Annenberg Center for Health Sciences at Eisenhower, Integritas, InThought, National Association for Continuing Education, Paradigm Medical Communications, LLC, PeerVoice, UpToDate, Haymarket Communications, Western Society of Allergy and Immunology, Proterixbio (formerly Bioscale), Unity Biotechnology, ConCert Pharmaceuticals, Lucid, Methodist Hospital, Columbia University, Prime Healthcare Ltd, WebMD, PeerView Network, California Society of Allergy and Immunology, Chiesi, Puerto Rico Thoracic Society, outside the submitted work. He is also a part of the event adjudication committee for Medtronic. SF has received grants from American Thoracic Society and Fisher & Paykel; personal fees from Hospital Medicine (SHM) and has consulted Genentech. The authors report no other conflicts of interest in this work.
Figures



References
-
- Soriano JB, Kendrick PJ, Paulson KR.; Collaborators GBDCRD. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8(6):585–596. doi:10.1016/S2213-2600(20)30105-3 - DOI - PMC - PubMed
-
- Global strategy for prevention, diagnosis and management of COPD: 2022 Report. GOLD Reports Web site. Available from: https://goldcopd.org/2022-gold-reports-2/. Accessed March 29, 2023.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

