Timosaponin AIII promotes non-small-cell lung cancer ferroptosis through targeting and facilitating HSP90 mediated GPX4 ubiquitination and degradation
- PMID: 37056925
- PMCID: PMC10086754
- DOI: 10.7150/ijbs.77979
Timosaponin AIII promotes non-small-cell lung cancer ferroptosis through targeting and facilitating HSP90 mediated GPX4 ubiquitination and degradation
Abstract
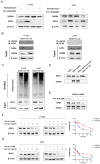
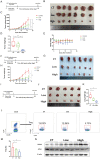
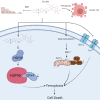
Timosaponin AIII (Tim-AIII), a steroid saponin, exhibits strong anticancer activity in a variety of cancers, especially breast cancer and liver cancer. However, the underlying mechanism of the effects of Tim-AIII-mediated anti-lung cancer effects remain obscure. In this study, we showed that Tim-AIII suppressed cell proliferation and migration, induced G2/M phase arrest and ultimately triggered cell death of non-small cell lung cancer (NSCLC) cell lines accompanied by the release of reactive oxygen species (ROS) and iron accumulation, malondialdehyde (MDA) production, and glutathione (GSH) depletion. Interestingly, we found that Tim-AIII-mediated cell death was reversed by ferroptosis inhibitor ferrostatin-1 (Fer-1). Meanwhile, the heat shock protein 90 (HSP90) was predicted and verified as the direct binding target of Tim-AIII by SwissTargetPrediction (STP) and surface plasmon resonance (SPR) assay. Further study showed that Tim-AIII promoted HSP90 expression and Tim-AIII induced cell death was blocked by the HSP90 inhibitor tanespimycin, indicating that HSP90 was the main target of Tim-AIII to further trigger intracellular events. Mechanical analysis revealed that the Tim-AIII-HSP90 complex further targeted and degraded glutathione peroxidase 4 (GPX4), and promoted the ubiquitination of GPX4, as shown by an immunoprecipitation, degradation and in vitro ubiquitination assay. In addition, Tim-AIII inhibited cell proliferation, induced cell death, led to ROS and iron accumulation, MDA production, GSH depletion, as well as GPX4 ubiquitination and degradation, were markedly abrogated when HSP90 was knockdown by HSP90-shRNA transfection. Importantly, Tim-AIII also showed a strong capacity of preventing tumor growth by promoting ferroptosis in a subcutaneous xenograft tumor model, whether C57BL/6J or BALB/c-nu/nu nude mice. Together, HSP90 was identified as a new target of Tim-AIII. Tim-AIII, by binding and forming a complex with HSP90, further targeted and degraded GPX4, ultimately induced ferroptosis in NSCLC. These findings provided solid evidence that Tim-AIII can serve as a potential candidate for NSCLC treatment.
Keywords: Timosaponin AIII; ferroptosis; glutathione peroxidase 4; heat shock protein 90; non-small cell lung cancer.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





References
-
- Global Cancer Observatory. cancer today. World Health Organization. International Agency for Research on Cancer.
-
- Barton MK. Encouraging long-term outcomes reported in patients with stage I non-small cell lung cancer treated with stereotactic ablative radiotherapy. CA Cancer J Clin. 2017;67:349–50. - PubMed
-
- Smith RA, Andrews KS, Brooks D, Fedewa SA, Manassaram-Baptiste D, Saslow D. et al. Cancer screening in the United States, 2019: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2019;69:184–210. - PubMed
-
- Arbour KC, Riely GJ. Systemic Therapy for Locally Advanced and Metastatic Non-Small Cell Lung Cancer: A Review. Jama. 2019;322:764–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

