Pomegranate peel extract protects against the development of diabetic cardiomyopathy in rats by inhibiting pyroptosis and downregulating LncRNA-MALAT1
- PMID: 37056985
- PMCID: PMC10086142
- DOI: 10.3389/fphar.2023.1166653
Pomegranate peel extract protects against the development of diabetic cardiomyopathy in rats by inhibiting pyroptosis and downregulating LncRNA-MALAT1
Abstract
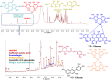
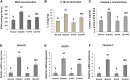
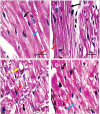
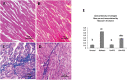
Background: Pyroptosis is an inflammatory programmed cell death accompanied by activation of inflammasomes and maturation of pro-inflammatory cytokines interleukin-1β (IL-1β) and IL-18. Pyroptosis is closely linked to the development of diabetic cardiomyopathy (DC). Pomegranate peel extract (PPE) exhibits a cardioprotective effect due to its antioxidant and anti-inflammatory properties. This study aimed to investigate the underlying mechanisms of the protective effect of PPE on the myocardium in a rat model of DC and determine the underlying molecular mechanism. Methods: Type 1 diabetes (T1DM) was induced in rats by intraperitoneal injection of streptozotocin. The rats in the treated groups received (150 mg/kg) PPE orally and daily for 8 weeks. The effects on the survival rate, lipid profile, serum cardiac troponin-1, lipid peroxidation, and tissue fibrosis were assessed. Additionally, the expression of pyroptosis-related genes (NLRP3 and caspase-1) and lncRNA-MALAT1 in the heart tissue was determined. The PPE was analyzed using UPLC-MS/MS and NMR for characterizing the phytochemical content. Results: Prophylactic treatment with PPE significantly ameliorated cardiac hypertrophy in the diabetic rats and increased the survival rate. Moreover, prophylactic treatment with PPE in the diabetic rats significantly improved the lipid profile, decreased serum cardiac troponin-1, and decreased lipid peroxidation in the myocardial tissue. Histopathological examination of the cardiac tissues showed a marked reduction in fibrosis (decrease in collagen volume and number of TGF-β-positive cells) and preservation of normal myocardial structures in the diabetic rats treated with PPE. There was a significant decrease in the expression of pyroptosis-related genes (NLRP3 and caspase-1) and lncRNA-MALAT1 in the heart tissue of the diabetic rats treated with PPE. In addition, the concentration of IL-1β and caspase-1 significantly decreased in the heart tissue of the same group. The protective effect of PPE on diabetic cardiomyopathy could be due to the inhibition of pyroptosis and downregulation of lncRNA-MALAT1. The phytochemical analysis of the PPE indicated that the major compounds were hexahydroxydiphenic acid glucoside, caffeoylquinic acid, gluconic acid, citric acid, gallic acid, and punicalagin. Conclusion: PPE exhibited a cardioprotective potential in diabetic rats due to its unique antioxidant, anti-inflammatory, and antifibrotic properties and its ability to improve the lipid profile. The protective effect of PPE on DC could be due to the inhibition of the NLRP3/caspase-1/IL-1β signaling pathway and downregulation of lncRNA-MALAT1. PPE could be a promising therapy to protect against the development of DC, but further clinical studies are recommended.
Keywords: IL1β; NLRP3; TGF-β; caspase-1; long non-coding RNA-metastasis-associated lung adenocarcinoma transcript-1; pomegranate peel extract.
Copyright © 2023 Abo-Saif, Ragab, Ibrahim, Abdelzaher, Mehanyd, Saber-Ayad and El-Feky.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Ahmed S. R., El-Sherei M. M., El-Dine R. S., El-Toumy S. A. (2019). Phytoconstituents, hepatoprotective, and antioxidant activities of Euphorbia cooperi N. E. Br. Egypt. J. Chem. 62, 831–840.
-
- Akuru E. A., Chukwuma C. I., Oyeagu C. E., Erukainure O. L., Mashile B., Setlhodi R., et al. (2022). Nutritional and phytochemical profile of pomegranate (“Wonderful variety”) peel and its effects on hepatic oxidative stress and metabolic alterations. J. Food Biochem. 46 (4), e13913. 10.1111/jfbc.13913 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

