A randomised, controlled, feasibility trial of an online, self-guided breathlessness supportive intervention (SELF-BREATHE) for individuals with chronic breathlessness due to advanced disease
- PMID: 37057089
- PMCID: PMC10086687
- DOI: 10.1183/23120541.00508-2022
A randomised, controlled, feasibility trial of an online, self-guided breathlessness supportive intervention (SELF-BREATHE) for individuals with chronic breathlessness due to advanced disease
Abstract
Introduction: SELF-BREATHE is a complex, transdiagnostic, supportive, digital breathlessness intervention co-developed with patients. SELF-BREATHE seeks to build capacity and resilience within health services by improving the lives of people with chronic breathlessness using nonpharmacological, self-management approaches. This study aimed to determine whether SELF-BREATHE is feasible to deliver and acceptable to patients living with chronic breathlessness.
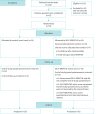
Methods: A parallel, two-arm, single-blind, single-centre, randomised controlled, mixed-methods feasibility trial with participants allocated to 1) intervention group (SELF-BREATHE) or 2) control group (usual National Health Service (NHS) care). The setting was a large multisite NHS foundation trust in south-east London, UK. The participants were patients living with chronic breathlessness due to advanced malignant or nonmalignant disease(s). Participants were randomly allocated (1:1) to an online, self-guided, breathlessness supportive intervention (SELF-BREATHE) and usual care or usual care alone, over 6 weeks. The a priori progression criteria were ≥30% of eligible patients given an information sheet consented to participate; ≥60% of participants logged on and accessed SELF-BREATHE within 2 weeks; and ≥70% of patients reported the methodology and intervention as acceptable.
Results: Between January 2021 and January 2022, 52 (47%) out of 110 eligible patients consented and were randomised. Of those randomised to SELF-BREATHE, 19 (73%) out of 26 logged on and used SELF-BREATHE for a mean±sd (range) 9±8 (1-33) times over 6 weeks. 36 (70%) of the 52 randomised participants completed and returned the end-of-study postal questionnaires. SELF-BREATHE users reported it to be acceptable. Post-intervention qualitative interviews demonstrated that SELF-BREATHE was acceptable and valued by users, improving breathlessness during daily life and at points of breathlessness crisis.
Conclusion: These data support the feasibility of moving to a fully powered, randomised controlled efficacy trial with minor modifications to minimise missing data (i.e. multiple methods of data collection: face-to-face, telephone, video assessment and by post).
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: C.C. Reilly received support for the present manuscript from the NIHR; and declares funding received from King's Together and Royal Brompton Hospital – King's Health Partnership Transformation, outside the submitted work. Conflict of interest: M. Maddocks has received grants or contracts from National Institute for Health Research (NIHR) Career Development Fellowship (CDF-2017–10-009) and NIHR Applied Research Collaboration South London (NIHR ARC South London) at King's College Hospital NHS Foundation Trust, outside the submitted work. Conflict of interest: T. Chalder receives salary support from the National Institute for Health Research (NIHR) Mental Health Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. The views expressed in this article are those of the authors and not necessarily those of the NIHR or the NHS. Conflict of interest: K. Bristowe has received grants or contracts from the National Institute for Health Research, Medical Research Council, Health Education England, European Commission, and Marie Curie, outside the submitted work. Conflict of interest: I.J. Higginson has received grants or contracts from the NIHR, UKRI, Cicely Saunders International and Marie Curie, outside the submitted work.
Figures
Similar articles
-
An online breathing and wellbeing programme (ENO Breathe) for people with persistent symptoms following COVID-19: a parallel-group, single-blind, randomised controlled trial.Lancet Respir Med. 2022 Sep;10(9):851-862. doi: 10.1016/S2213-2600(22)00125-4. Epub 2022 Apr 27. Lancet Respir Med. 2022. PMID: 35489367 Free PMC article. Clinical Trial.
-
Guided self-help for depression in autistic adults: the ADEPT feasibility RCT.Health Technol Assess. 2019 Dec;23(68):1-94. doi: 10.3310/hta23680. Health Technol Assess. 2019. PMID: 31856942 Free PMC article. Clinical Trial.
-
Helping Patients with COPD Transition from Hospital to Home—The BREATHE Study [Internet].Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2020 Apr. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2020 Apr. PMID: 39680694 Free Books & Documents. Review.
-
"The whole of humanity has lungs, doesn't it? We are not all the same sort of people": patient preferences and choices for an online, self-guided chronic breathlessness supportive intervention: SELF-BREATHE.ERJ Open Res. 2022 Jul 11;8(3):00093-2022. doi: 10.1183/23120541.00093-2022. eCollection 2022 Jul. ERJ Open Res. 2022. PMID: 35821758 Free PMC article.
-
Digital interventions for hypertension and asthma to support patient self-management in primary care: the DIPSS research programme including two RCTs [Internet].Southampton (UK): National Institute for Health and Care Research; 2022 Dec. Southampton (UK): National Institute for Health and Care Research; 2022 Dec. PMID: 36538606 Free Books & Documents. Review.
Cited by
-
Illness perceptions, cognitive and behavioural responses to chronic breathlessness in individuals living with advanced respiratory disease: an observational study.ERJ Open Res. 2024 Apr 29;10(2):00874-2023. doi: 10.1183/23120541.00874-2023. eCollection 2024 Mar. ERJ Open Res. 2024. PMID: 38686180 Free PMC article.
-
Multicomponent services for symptoms in serious respiratory illness: a systematic review and meta-analysis.Eur Respir Rev. 2024 Oct 30;33(174):240054. doi: 10.1183/16000617.0054-2024. Print 2024 Oct. Eur Respir Rev. 2024. PMID: 39477352 Free PMC article.
-
Recent advances in understanding the role of antidepressants to manage breathlessness in supportive and palliative care.Curr Opin Support Palliat Care. 2025 Jun 1;19(2):83-94. doi: 10.1097/SPC.0000000000000761. Epub 2025 Apr 21. Curr Opin Support Palliat Care. 2025. PMID: 40265531 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources