Adventitial adaptive immune cells are associated with ascending aortic dilatation in patients with a bicuspid aortic valve
- PMID: 37057097
- PMCID: PMC10086356
- DOI: 10.3389/fcvm.2023.1127685
Adventitial adaptive immune cells are associated with ascending aortic dilatation in patients with a bicuspid aortic valve
Abstract
Background: Bicuspid aortic valve (BAV) is associated with ascending aorta aneurysms and dissections. Presently, genetic factors and pathological flow patterns are considered responsible for aneurysm formation in BAV while the exact role of inflammatory processes remains unknown.
Methods: In order to objectify inflammation, we employ a highly sensitive, quantitative immunohistochemistry approach. Whole slides of dissected, dilated and non-dilated ascending aortas from BAV patients were quantitatively analyzed.
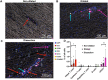
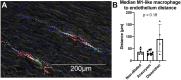
Results: Dilated aortas show a 4-fold increase of lymphocytes and a 25-fold increase in B lymphocytes in the adventitia compared to non-dilated aortas. Tertiary lymphoid structures with B cell follicles and helper T cell expansion were identified in dilated and dissected aortas. Dilated aortas were associated with an increase in M1-like macrophages in the aorta media, in contrast the number of M2-like macrophages did not change significantly.
Conclusion: This study finds unexpected large numbers of immune cells in dilating aortas of BAV patients. These findings raise the question whether immune cells in BAV aortopathy are innocent bystanders or contribute to the deterioration of the aortic wall.
Keywords: aortic dissection; auto-inflammation; bicuspid aortc valve; inflammation; multiplex immunohistochemistry; tertiary lymphoid structures; thoracic aorta aneurysm.
Copyright © 2023 Staal, Cortenbach, Gorris, van der Woude, Srinivas, Heijmen, Geuzebroek, Grewal, Hebeda, de Vries, DeRuiter and van Kimmenade.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Dysregulation of Endothelial Nitric Oxide Synthase Does Not Depend on Hemodynamic Alterations in Bicuspid Aortic Valve Aortopathy.J Am Heart Assoc. 2020 Sep 15;9(18):e016471. doi: 10.1161/JAHA.120.016471. Epub 2020 Sep 2. J Am Heart Assoc. 2020. PMID: 32873108 Free PMC article.
-
Seno-destructive smooth muscle cells in the ascending aorta of patients with bicuspid aortic valve disease.EBioMedicine. 2019 May;43:54-66. doi: 10.1016/j.ebiom.2019.04.060. Epub 2019 May 9. EBioMedicine. 2019. PMID: 31078518 Free PMC article.
-
Impaired collagen biosynthesis and cross-linking in aorta of patients with bicuspid aortic valve.J Am Heart Assoc. 2013 Feb 14;2(1):e000034. doi: 10.1161/JAHA.112.000034. J Am Heart Assoc. 2013. PMID: 23525417 Free PMC article.
-
Bicuspid Aortic Valves: an Up-to-Date Review on Genetics, Natural History, and Management.Curr Cardiol Rep. 2022 Aug;24(8):1021-1030. doi: 10.1007/s11886-022-01716-2. Epub 2022 Jul 22. Curr Cardiol Rep. 2022. PMID: 35867195 Review.
-
Normal and abnormal development of the aortic valve and ascending aortic wall: a comprehensive overview of the embryology and pathology of the bicuspid aortic valve.Ann Cardiothorac Surg. 2022 Jul;11(4):380-388. doi: 10.21037/acs-2021-bav-14. Ann Cardiothorac Surg. 2022. PMID: 35958528 Free PMC article. Review.
Cited by
-
Immunotherapy in the Context of Aortic Valve Diseases.Cardiovasc Drugs Ther. 2024 Dec;38(6):1173-1185. doi: 10.1007/s10557-024-07608-7. Epub 2024 Jul 17. Cardiovasc Drugs Ther. 2024. PMID: 39017904 Free PMC article. Review.
References
-
- Borger MA, Fedak PWM, Stephens EH, Gleason TG, Girdauskas E, Ikonomidis JS, et al. . The American Association for Thoracic Surgery consensus guidelines on bicuspid aortic valve–related aortopathy: executive summary. J Thorac Cardiovasc Surg. (2018) 156:473–80. doi: 10.1016/j.jtcvs.2017.10.161, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

